Autophagy more than a cytosolic event
Autophagy is a cellular process whereby cytosolic components are broken down and eliminated or recycled. As a homeostatic mechanism, basal autophagic activity eliminates excess or abnormal proteins and organelles1. As an induced process, autophagy may be triggered by various external challenges, such as decreased nutrient and energy resources, and oxidative stress1.
During autophagy, several cytosolic ATG (autophagy-related) and ATG-associated proteins drive the formation of the engulfing organelle1,2. ATGs play key roles in the formation of the initial membrane vesicle or phagophore, and its subsequent elongation leading to the engulfment of cellular components. The resulting autophagosome fuses with lysosomes allowing the degradation of the sequestered content1.
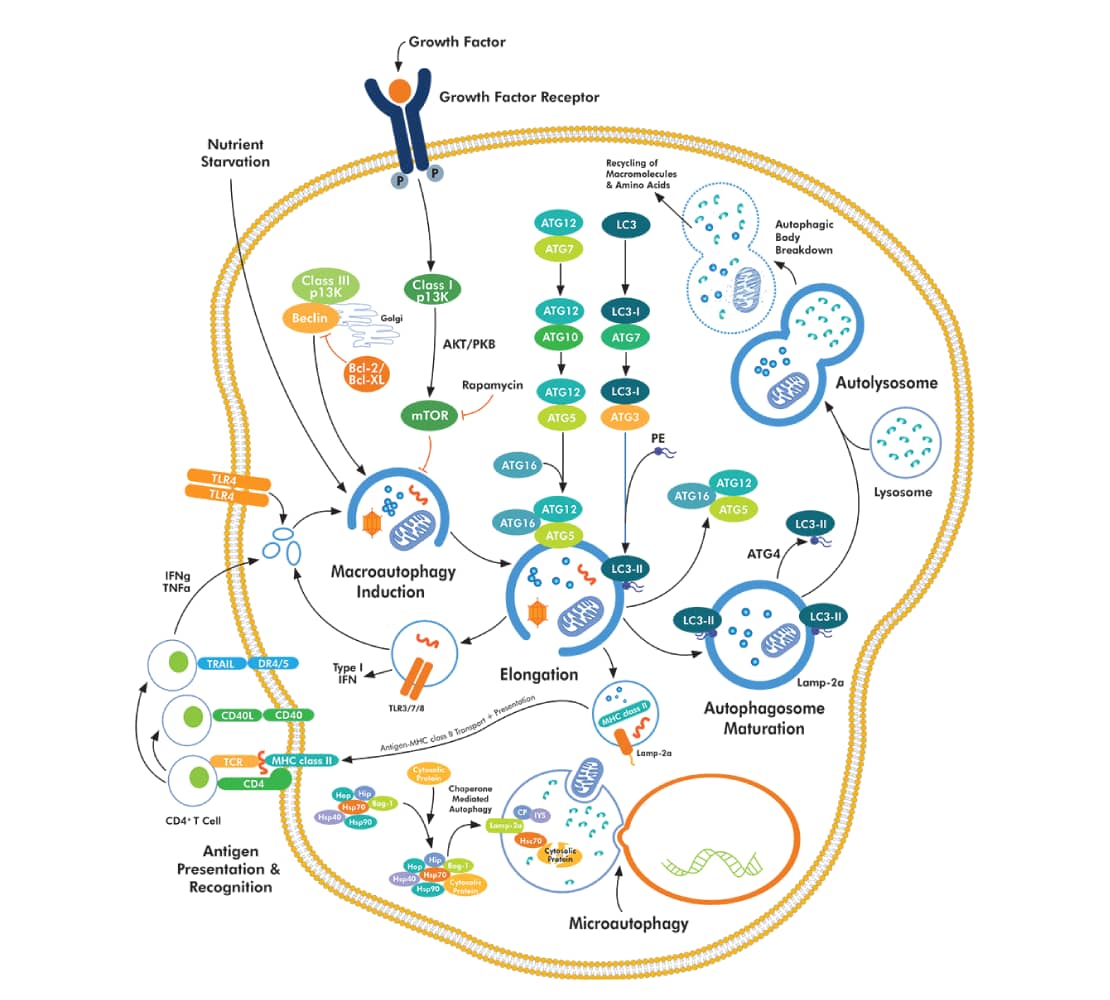
ATG (autophagy-related) and ATG-associated proteins drive the formation of the engulfing organelle1,2. ATGs play key roles in the formation of the initial membrane vesicle or phagophore, and its subsequent elongation leading to the engulfment of cellular components. The resulting autophagosome fuses with lysosomes allowing the degradation of the sequestered content1.
Autophagy has been considered to occur mostly under cytosolic control2. Nevertheless, new findings provide evidence for the role of both cytoplasmic and nuclear mechanisms in the regulation of autophagy2.
“As shown in one of our papers regarding methylation and aging, it seems that the promoter regions of autophagy genes are more methylated in aging mice. Demethylation agents were able to improve autophagy in this model. But is it safe to use this strategy in aging population? or should we target only those with early stages of Alzheimer’s, Parkinson’s and Huntington’s disease?
My lab obtained samples from human brains who passed away from Alzheimer’s disease and we are analyzing several epigenetic parameters to determine the underlying mechanisms associated with the decline of autophagy activity in the human brain. More studies are needed to answer these questions.”
Professor Amal Amer, MD,PhD
Department of Microbial Infection and Immunity
Center for Microbial Interface Biology
Ohio State University
Epigenetic control of autophagy
Recently, the role of epigenetic mechanisms in regulating autophagy has come to the forefront of research in this field. Several studies support a role for epigenetic changes, such as DNA methylation and histone modifications, in the regulation of autophagy1, 2,3,. A recent study focused on the relationship between aging and autophagy3. Senescence is associated with decreased autophagy, which prompted investigators to explore the underlying mechanism. Their findings revealed that in immune cells from aged mice, decreased autophagic activity is associated with a decrease in the expression of autophagy genes3. Moreover, decreased expression of autophagy genes occurred as the result of their increased methylation, likely mediated by the DNA methyltransferase DNMT23. Investigators identified the gene encoding ATG5, an autophagy protein involved in the elongation of autophagosomes, as one of the highly methylated autophagy genes3.
Several histone modifications have been recently associated with the regulation of autophagy. Histones, the main protein associated with chromosomal DNA, may be modified by a series of enzymes that induce their phosphorylation, methylation, acetylation or ubiquitination1. These modifications or “histone marks” are known to influence the chromatin’s structure and gene expression, and thus impact overall function1. Recently, the monoubiquitination of histone H2B was shown to play a role in autophagy regulation4. Loss of H2B monoubiquitination leads to increased autophagy. Moreover, consistent with this finding, decreased nutrient availability is associated with a decrease in H2B monoubiquitination4.
Overall, these recent findings further highlight the importance that nuclear mechanisms play in the regulation of autophagy.
-
Mizushima N. (2007) Autophagy: process and function. Genes & Dev. 21:2861-287.
-
Füllgrabe J. et al. (2014) The return of the nucleus: transcriptional and epigenetic control of autophagy. Nature Reviews Molecular Cell Biology. 15:65-74.
-
Khalil H. et al. (2016) Aging is associated with hypermethylation of autophagy genes in macrophage. Epigenetics. 11:381-388.
-
Chen S. et al. (2017) Histone H2B monoubiquitination is a critical epigenetic switch for the regulation of autophagy. Nucleic Acids Research. 43:1144-1158.