ELISA Illustrated Assay
Learn About:
Overview
ELISA Theory
- ELISA is actually an acronym: Enzyme-Linked ImmunoSorbent Assay.
- Similar to Western Blots, antibodies are used to detect the presence of proteins or other antibodies, known as 'antigens'.
- Unlike Western Blots, the protein or antibody is “bound” to a well, and hundreds of samples can be analyzed quickly.
ELISA Types
There are three major types of ELISAs: Indirect, Sandwich and Competitive.
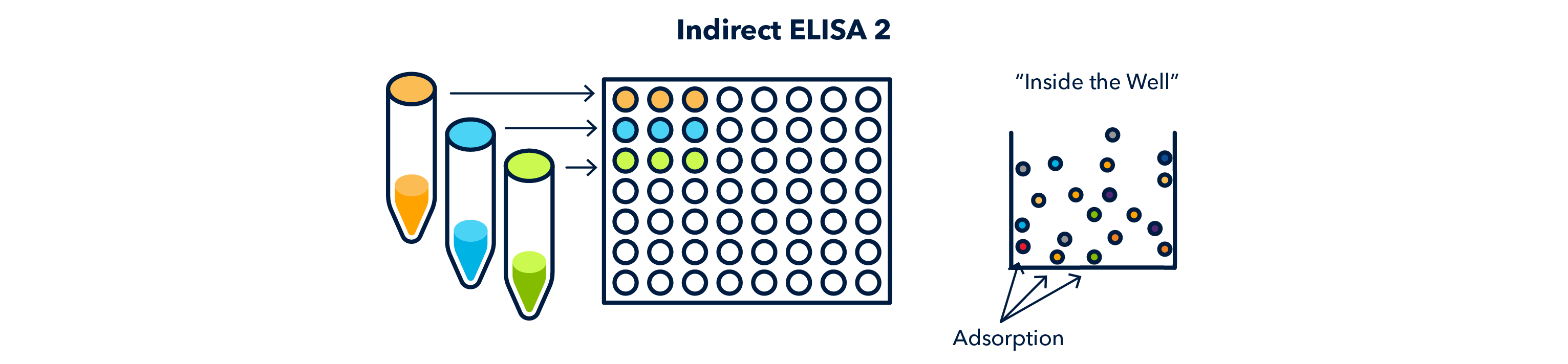
- Indirect: the protein sample is bound through adsorption, directly (and non-specifically) to the well. Next, an antibody is used to detect the presence of one of the proteins contained in the sample, known as the antigen.
- Sandwich: a 'capture' antibody is bound to the well first, and when the sample is added, only proteins the antibody recognizes are 'captured'. Next, a second 'detection' antibody is used to detect the bound protein. The 'capture' and 'detection' antibodies are commonly called 'matched-pairs'. Finally, a third, enzyme-labeled antibody is added to detect the 'detection' antibody.
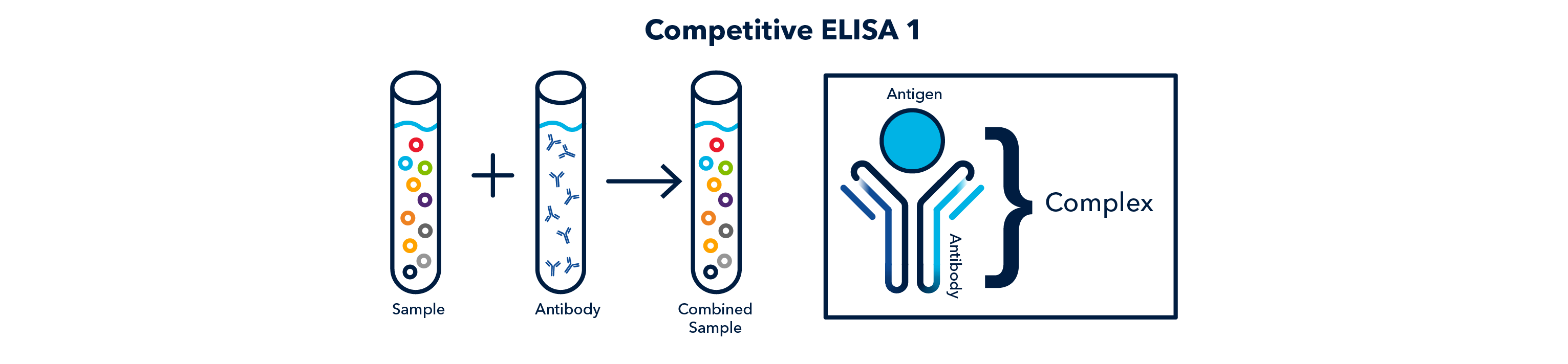
- Competitive: a primary antibody is incubated with the sample, which forms a complex. The complex is then adsorbed to the wells. Next, a secondary antibody is added to the wells, which recognizes the primary antibody only if it is not bound to the antigen. Therefore, the secondary antibody 'competes' with the antigen.
Samples are then added to the wells of a plate suitable for antigen binding and incubated so that the antigen will be thoroughly adsorbed to the well surface.
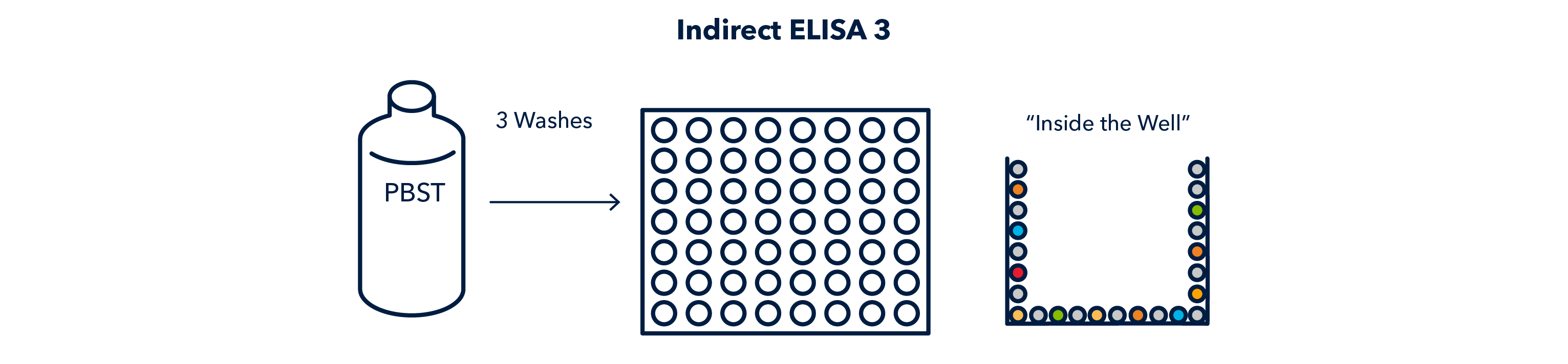
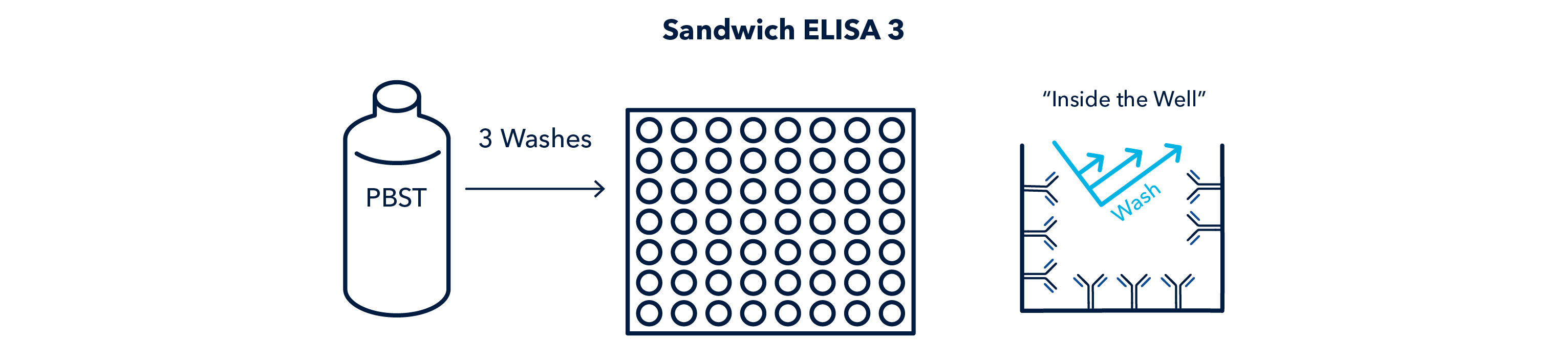
After incubation, the wells must be washed to remove any unbound, or poorly bound antigen. This step is very important and will occur after every other step.
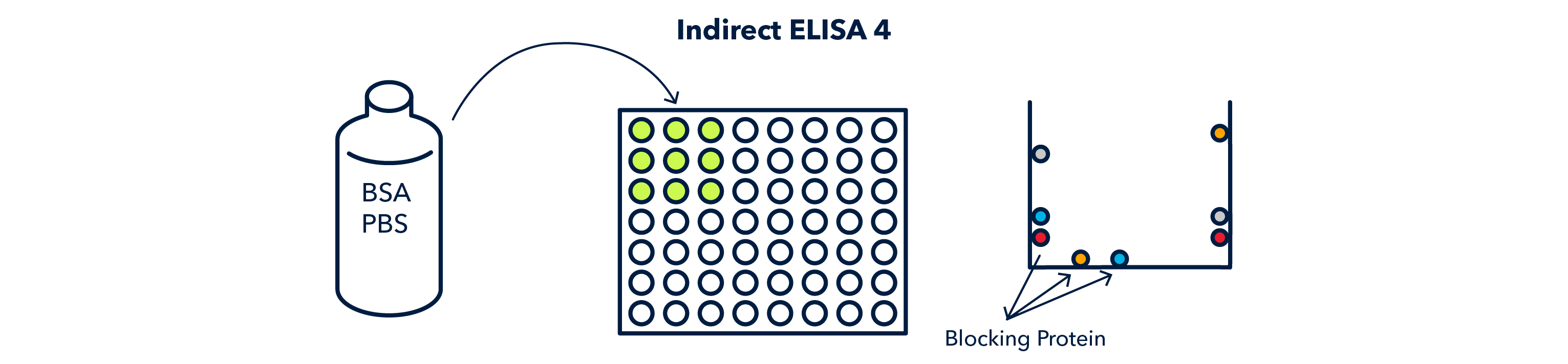
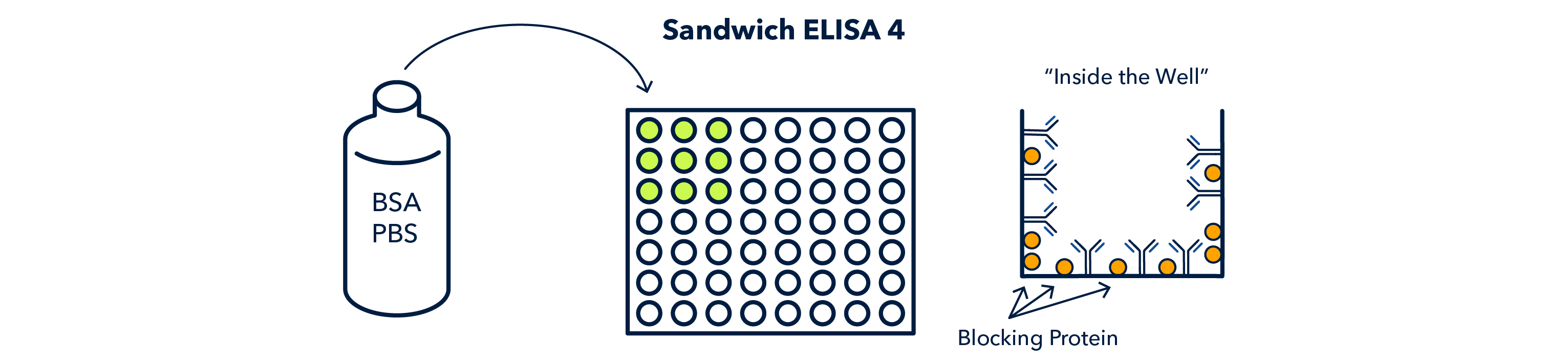
Next, the wells must be filled with 'blocking' solution, which contatins a nonspecific protein that binds to the exposed surfaces in the well and keeps the primary antibody from binding non-specifically to the well.
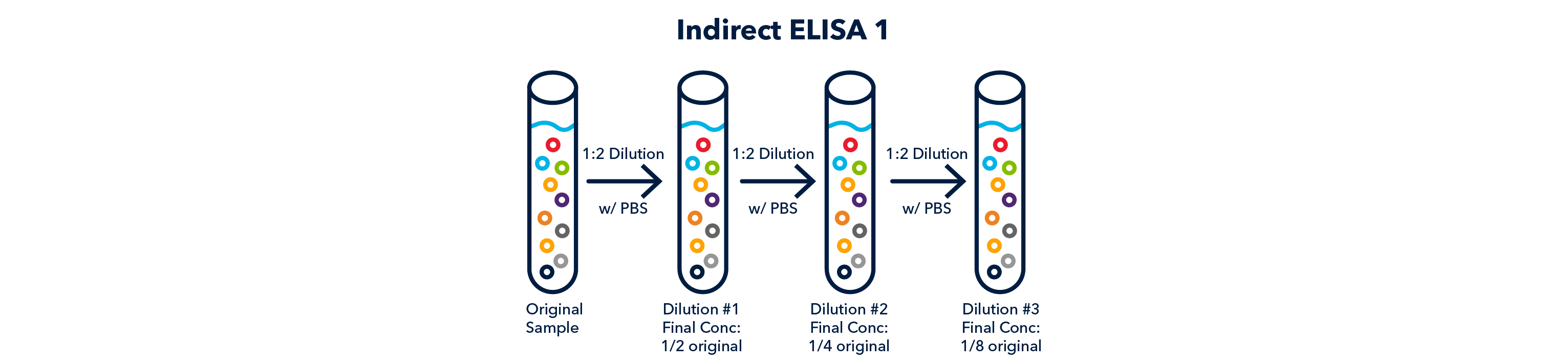
Following blocking, the plate is washed again and the primary antibody, in dilution, is added to the well. The antibody will only recognize one antigen, ideally. The primary antibody is usually added in a range of dilutions, and each dilution is usually tested in duplicate or triplicate.
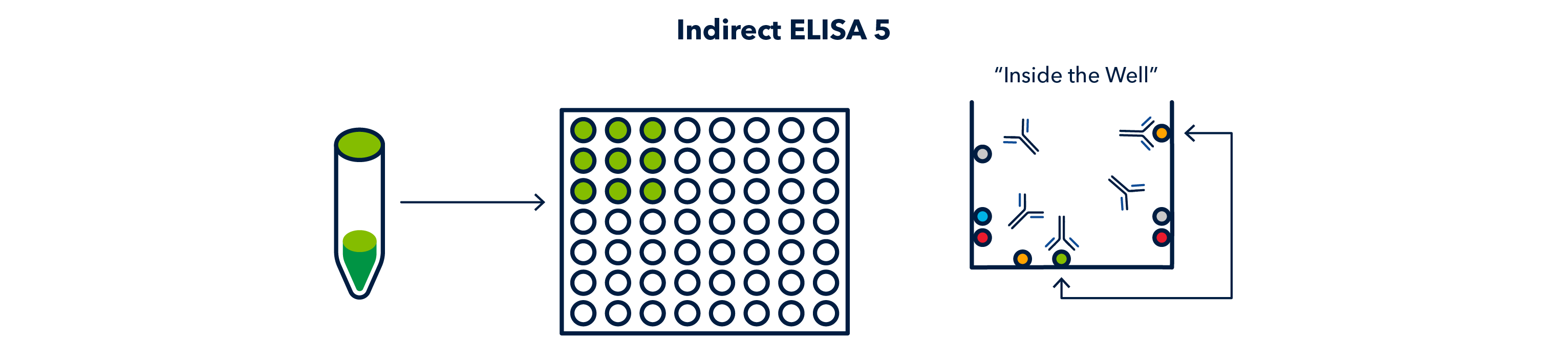
After primary antibody incubation, another series of washes are performed and then conjugated secondary antibody is added to each well, at a constant dilution.
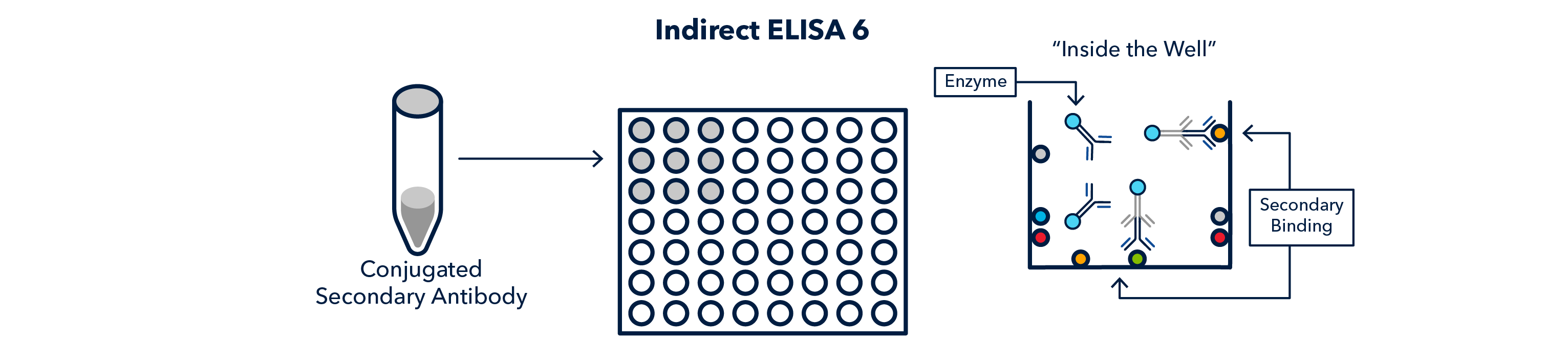
Finally, the wells are washed again, and the enzyme-conjugated secondary antibody is caused to react, giving off a signal, which is read on a 'plate reader'. The 'substrate' used varies according to the enzyme the secondary antibody is conjugated to.
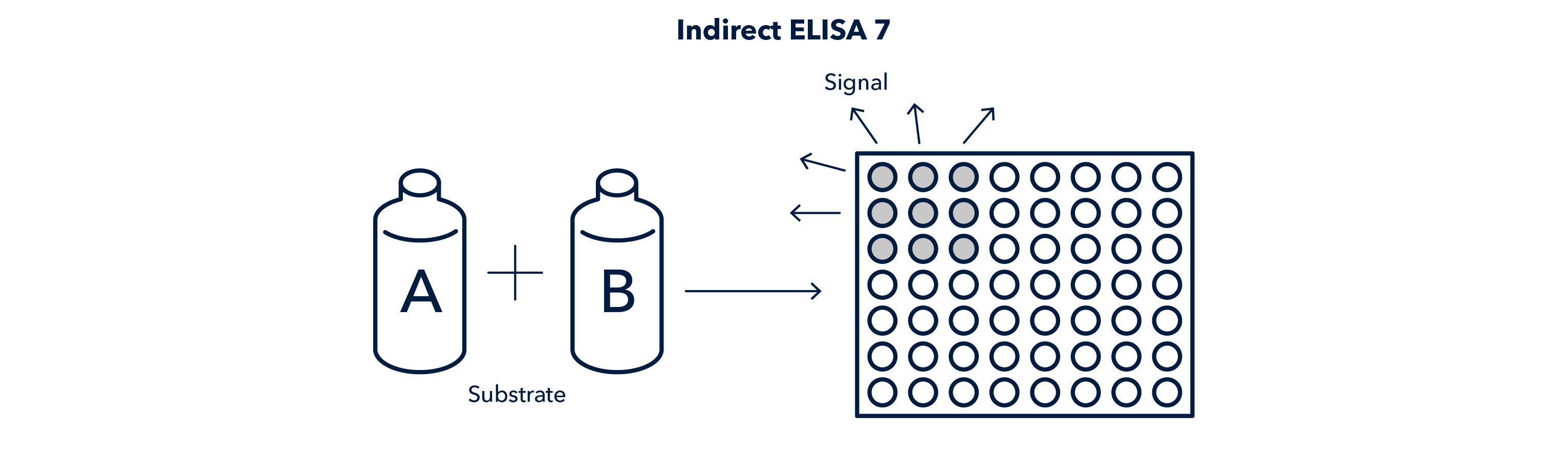
Indirect ELISA Results
- The presence of a signal from the secondary antibody means the antigen of interest is present.
- In order to determine antigen concentration, a standard curve of known antigen concentration must be run on the same plate.
- Negative controls should also be run to make sure your antibodies are binding specifically to the antigen only.
Indirect ELISA Pros/Cons
- This type of ELISA is commonly used to determine the ideal concentration/dilution of primary antibody to use in other experiments.
- This is done by running the ELISA against a known concentration of antigen, and performing serial dilutions of the primary antibody.
- The dilutions that result in a signal, show the range the primary antibody is effective in, according to the concentration of the antigen.
- Because the desired antigen may be present in extremely small quantities relative to the presence of other proteins, and because all proteins in a sample will bind to the well, Indirect ELISA may be unable to detect the presence of antigen in a sample.
- In this case, the antigen is usually purified out of the sample, so that it can be more readily detected.
- The primary and secondary antibodies used in this type of ELISA can also be used in the same dilutions against the same samples in Western Blot, therefore eliminating the need to purchase and test other antibodies.
The 'capture' antibody, also known as the first primary antibody, is added to each well, and incubated, to allow the antibody to adsorb to the surface of the well.
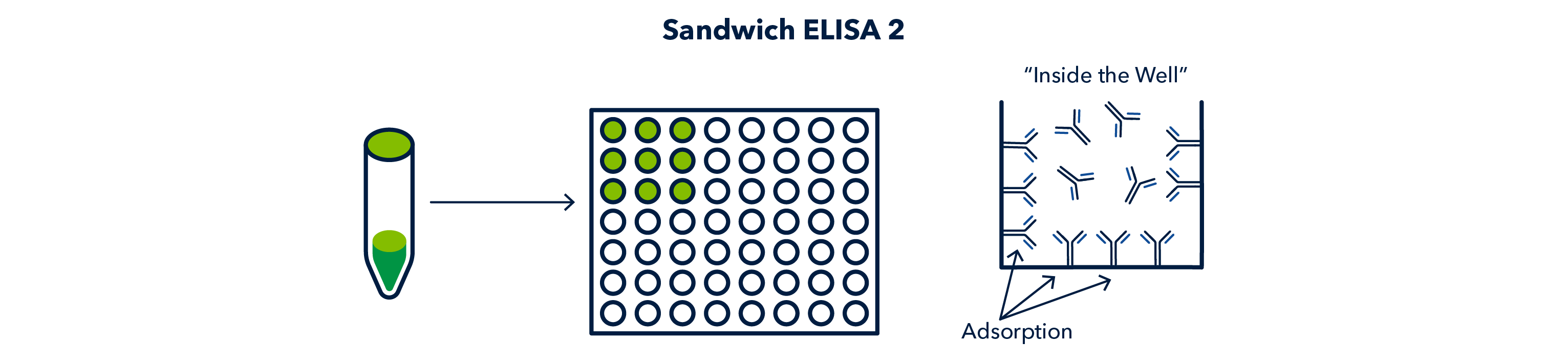
After incubation of the capture antibody, the wells are washed to remove any unbound antibody.
Next, 'blocking' solution is added. This is non-specific protein that binds to the open sites in the well and keeps the non-specific proteins in the sample from binding to the well.
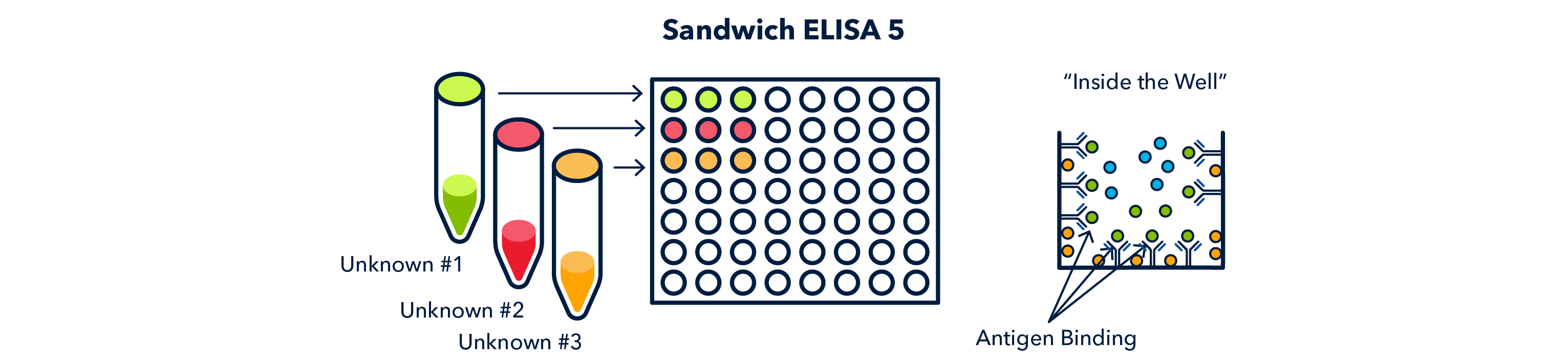
Next, samples of unknowns are added to each antibody-coated well and again allowed to incubate and bind to the capture antibody.
After another wash cycle, the 'detection' antibody is added to the wells and allowed to incubate and detect the antigen. The detection antibody must be a 'matched-pair' with the capture antibody, to make sure that the antibodies don't recognize each other, or the same site on the antigen of interest.
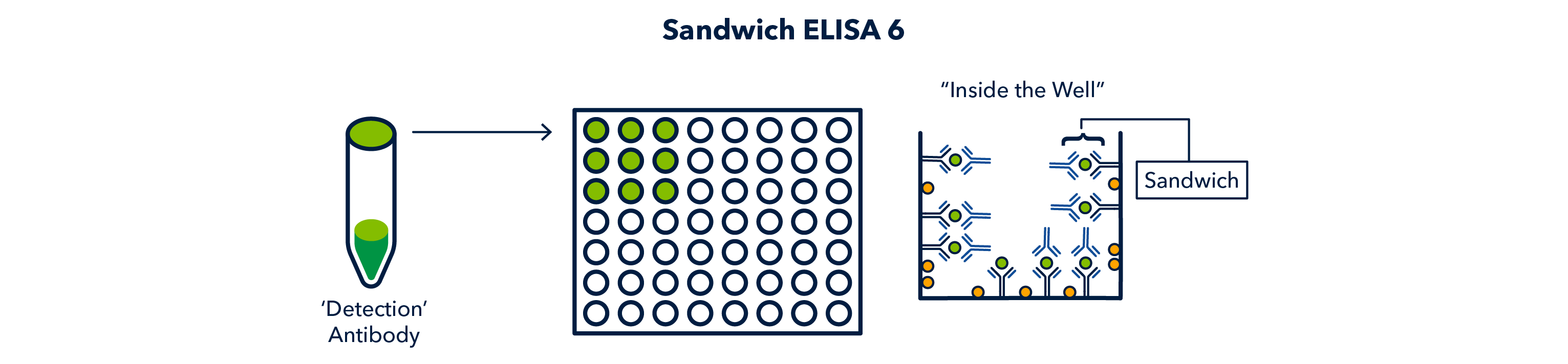
After another wash, the enzyme-linked antibody (sometimes referred to as a secondary, even though it is tertiary in this case) is added to each well and it detects the 'detection' antibody.
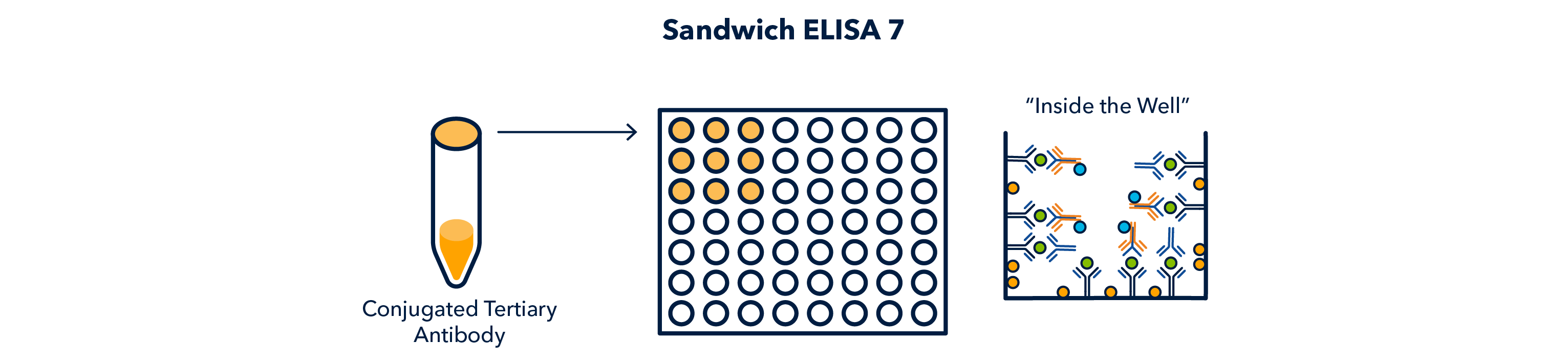
Finally, another wash cycle is performed, and the enzyme on the tertiary antibody is reacted, to give off a signal, which can be read on a plate reader.

Sandwich ELISA Results
- The presence of signal means the antigen of interest is present.
- A standard curve must be included on the same plate to make this ELISA quantitative.
- Negative controls should also be run to make sure only specific binding is occurring among the many antibodies used.
Sandwich ELISA Pros/Cons
- Unlike Indirect ELISAs, antigens of very low or unknown concentration in the sample can be detected because the 'capture' antibody only grabs the antigen of interest and all the other proteins in the sample are washed away.
- It is not necessary to use a tertiary enzyme-linked antibody, if the 'detection' antibody is already enzyme-linked. However, it can be very difficult, if not impossible to find a matched-pair where the 'detection' antibody is already conjugated.
- The end-user can conjugate the 'detection' antibody themselves, if so inclined.
- Only monoclonal antibodies can be used as matched pairs, because only monoclonals recognize one specific site on an antigen (known as the 'epitope').
- Monoclonal antibodies can be more expensive than polyclonal antibodies and matched-pair antibodies can be very difficult to find.
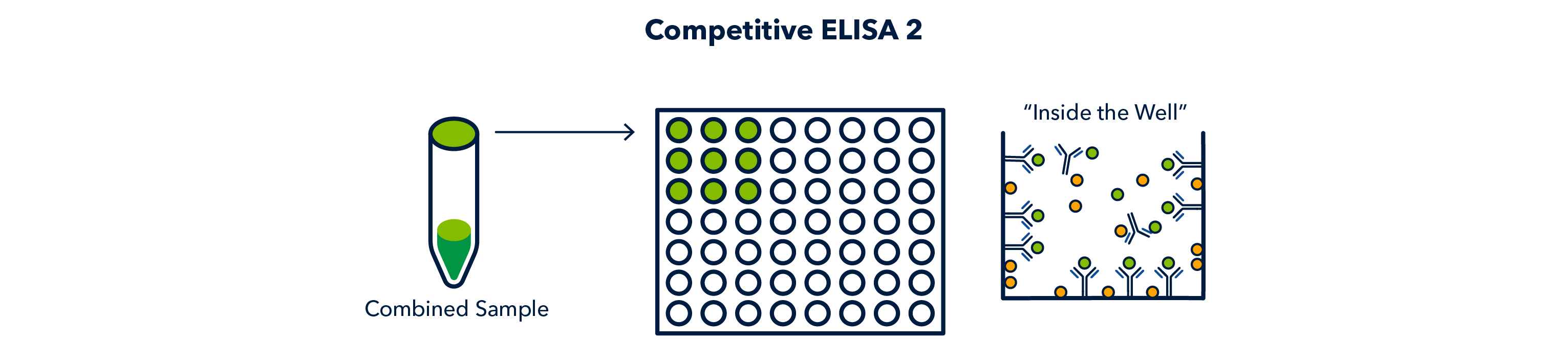
The sample is added to the well, and allowed to incubate so that it adsorbs to the surface of the well. The 'complex', any unbound antibody, and other proteins can all adsorb.
After sample incubation, the wells are washed to remove any unbound protein or antibody/antigen 'complex'.

The wells are then coated with blocking solution, which will keep the secondary antibody from non-specifically binding to the wells.
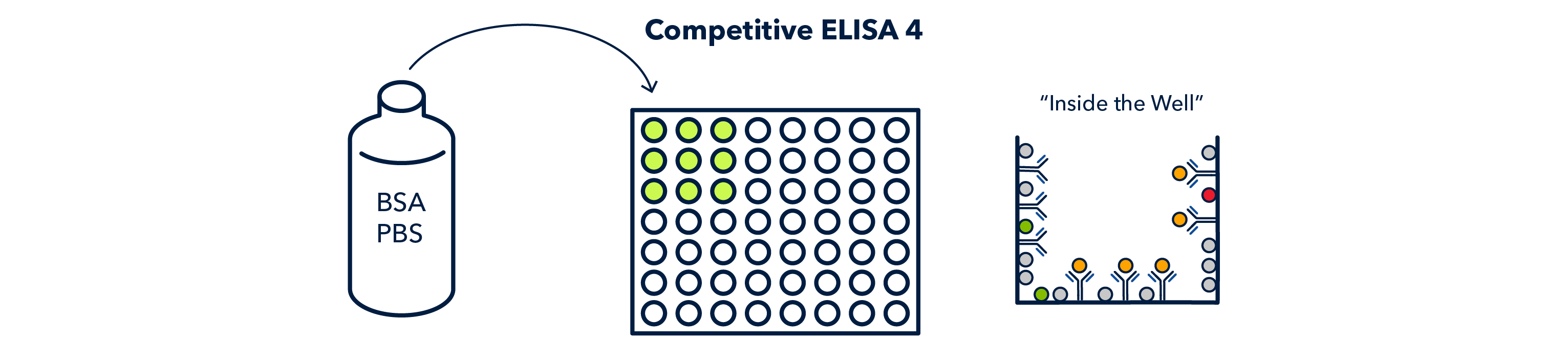
After another wash cycle, the conjugated secondary antibody is allowed to incubate and 'compete' with the antigen of interest for continued binding to the primary antibody.
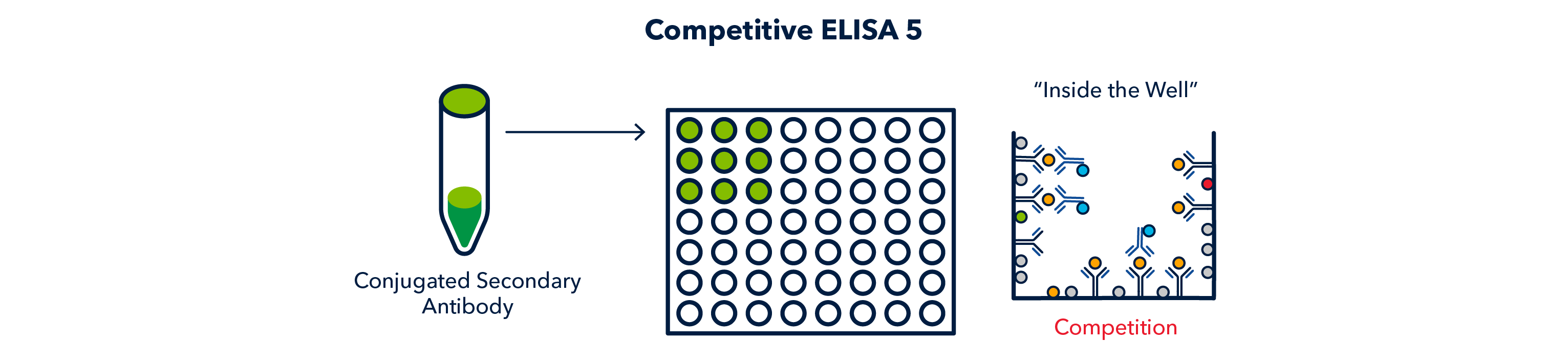
After a final wash cycle, the conjugated secondary enzyme is reacted to produce a signal, which can be read on a plate reader.

Competitive ELISA Results
- The strength of the signal is inversely related to the quantity of antigen present. The more antigen present, the more difficult it is for the secondary antibody to bind to the primary antibody and vice versa.
- Just as in other ELISAs, known standards can be run to determine concentration, but remember the inverse rule.
- Like Sandwich ELISA, this form of ELISA can detect smaller quantities of antigen present than Indirect ELISAs can.
- This type of ELISA does not require the use of matched-pair antibodies, as Sandwich ELISAs do.
- Instead of a conjugated secondary antibody being used as the competitor, another conjugated antigen can be used that the primary antibody also recognizes.
- In other words, use a protein that the primary antibody also recognizes, that is not the same as the antigen of interest.
- The benefit of using this method is that you do not have to use a secondary antibody.
- The disadvantage is that you may have difficulty finding another protein your primary antibody recognizes, and you will also likely have to conjugate the protein yourself.
- The primary antibody used can be unpurified, and polyclonal.
- The antigen of interest that is ideal for this type of ELISA contains only one recognizable epitope by the primary antibody.
Common Problems
- The negative controls can give positive results when the blocking solution isn't effective, therefore the secondary antibody or antigen of interest can bind to the open sites in the well.
- If the positive controls or standards give no signal, check your chemicals and be aware that the enzyme reaction is short-term, so the plate should be read as quickly as possible.
- Running your samples in duplicate and triplicate will allow for a more accurate determination of concentration.
- Applying your primary antibody in a dilution range increases the likelihood that you will get a signal that is neither too weak nor too strong.
- Past a certain limit, the strength of a signal gives useless information. Dilute your sample or primary antibody if this occurs.