Western Blot SDS-Page
Loading and Running the Gel
After sample preparation, samples in loading buffer must be loaded onto a gel. Proteins in the sample are separated from each other based on their size by SDS-PAGE gel electrophoresis. Electrophoresis is performed with a negative pole (cathode) on one end of the gel and a positive pole (anode) on the opposite end of the gel. The negatively charged SDS bound to proteins causes migration of protein complexes towards the positive pole (anode) during electrophoresis, allowing proteins to be separated by size. In general, the larger the protein, the slower it migrates through the gel. Acrylamide gels can be prepared at different concentrations. As a general rule, low molecular weight proteins are best resolved on high percentage gels, whereas large proteins require lower percentage gels for sufficient resolution.
With our automated Simple Western™ instruments, all steps following sample preparation and plate loading are automated. Simple Western uses capillary electrophoresis to separate your samples and then your samples are covalently immobilized to the capillary wall. The steps of antibody binding and detection are precisely controlled inside an automated benchtop instrument, providing fully quantitative and reproducible results with flexible multiplex detection strategies and total protein normalization.
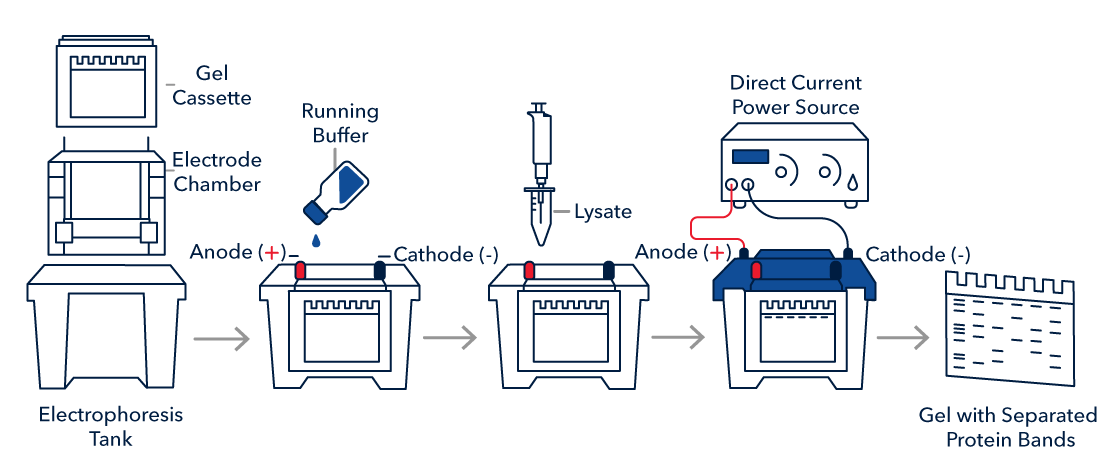
SDS-PAGE Gel Electrophoresis Protocol
1. Prepare or purchase a pre-made gel of appropriate polyacrylamide percentage to best resolve your protein of interest based on molecular weight.
| Buffer | Components |
|---|---|
| 1X Running buffer | 25 mM Tris base 192 mM glycine 0.1% SDS Adjust pH to 8.3 |
4. Run the gel as recommended by the manufacturer.
Note: 1-2 hours at 100 V is standard, but time and voltage may require optimization.