| Key Benefits: |
- Utilizes our highest quality FBS
- Extended testing on mouse embryonic stem (mES) cells
- Screened for colony morphology, pluripotency, and plating efficiency
- Maintains mES cells in an undifferentiated state
- Low endotoxin and hemoglobin levels
- USDA-Approved FBS of Mexico/Central American origin
- Manufactured in our ISO 9001:2015 certified facility
- Formerly sold under the Atlanta Biologicals brand
Summary for Fetal Bovine Serum - Embryonic Stem Cell Qualified
Why Use Embryonic Stem Cell Qualified Fetal Bovine Serum?
Only the highest quality Fetal Bovine Serum, also known as Fetal Calf Serum (FCS), lots are selected for further testing regarding suitability for culturing embryonic stem cells. This screening includes plating efficiency, colony morphology, and cytotoxicity assays. ES Qualified FBS lots must offer outstanding performance with embryonic stem cells while maintaining the cells in their valuable undifferentiated state. All lots of Embryonic Stem Cell Qualified FBS are manufactured in our ISO 9001:2015 certified facility.
Fetal Bovine Serum and other serum products should be stored and handled correctly to assure long-term stability and to preserve growth performance consistency throughout its shelf-life. In addition, heat inactivation of serum is frequently desired to inactivate complement within the serum. Below are protocol links for serum storage/handling and heat inactivation.
Protocol for Fetal Bovine Serum Storage, Thawing, and Freezing.
Protocol for Heat Inactivation of Serum Products.
Collection, Processing, and Testing of R&D Systems FBS
Collection and Processing - R&D Systems FBS is manufactured under a process that meets USDA requirements for animal products and ensures the product is traceable back to the date and location of collection. Our FBS manufacturing adheres to closed-system collection, processing in positive pressure, HEPA-filtered clean rooms, and triple 0.1 um filtration to remove contaminants. Strict control of the process from collection through processing enables us to provide a stable, consistent performing, and traceable supply of quality serum throughout global fluctuations in serum availability and regulatory requirements.
Virus Testing - Fetal Bovine Serum – Embryonic Stem Cell Qualified undergoes testing for adventitious viruses, such as: Bovine Viral Diarrhea Virus (BVDV), Infectious Bovine Rhinotracheitis Virus (IBRV), Parainfluenza-3 Virus (PI-3V), Bluetongue Virus (BTV), Bovine Respiratory Syncytial Virus (BRSV), Bovine Parvovirus (BPV), Bovine Adenovirus, Type 3 (BAV-3), Bovine Adenovirus, Type 5 (BAV-5), Reo virus, and Rabies virus.
Biochemical Testing - R&D Systems FBS undergo biochemical profiling including total protein, albumin, globulin, IgG, total bilirubin, glucose, iron, cholesterol, triglycerides, sodium and potassium. View the product insert for a complete list of biochemical profiling components, which may vary between grades.
Chemical Analysis - Each lot of FBS is tested for osmolality, pH, hemoglobin, endotoxin (LAL method) and microbiological contamination (using methods recommended by the U.S. Pharmacopeia).
Product Specifications for Fetal Bovine Serum - Embryonic Stem Cell Qualified
| Species |
Bovine
|
| Applications |
Cell Culture
|
| Formulation |
Liquid
|
| Purification |
Triple 0.1 μm filtered
|
| Osmolality |
280 -335 mOsm
|
| Sterility Testing |
Pass
|
| Quality Testing |
Pass
|
| Additional Testing |
pH - 6.8-7.8
Endotoxin - ≤ 50.0 EU/mL by Limulus Amebocyte Lysate (LAL) Assay
Hemoglobin - 25 mg/dL
Embryonic Stem Cell Culture - Plating efficacy, colony formation, and toxicity
|
Scientific Data Examples for Fetal Bovine Serum - Embryonic Stem Cell Qualified
|
|
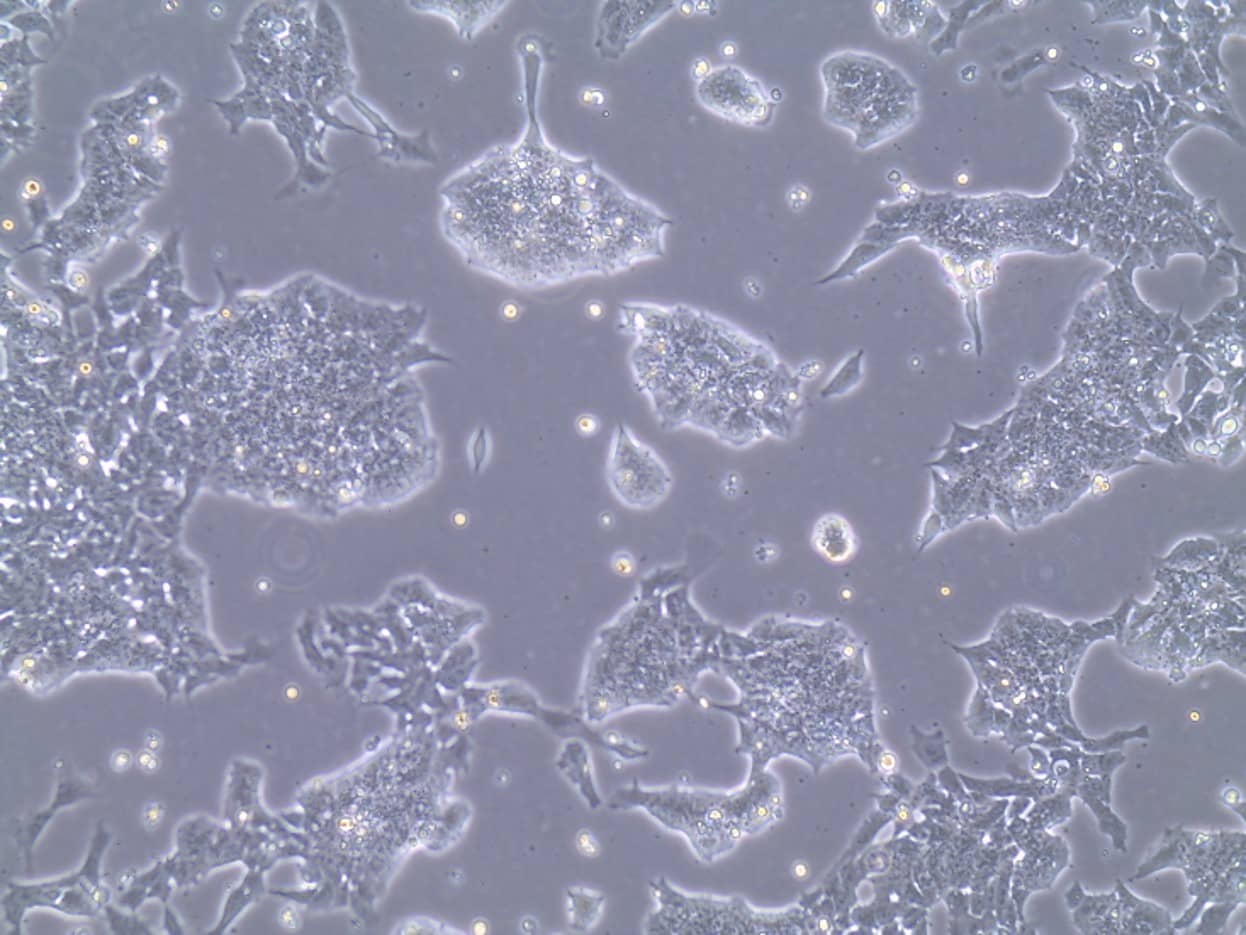
D3 Mouse Embryonic Stem Cells Grown in FBS - ESC Qualified.
Representative brightfield image showing colony formation of D3 mouse embryonic stem cells cultured using FBS - Embryonic Stem Cell Qualified from R&D Systems for 4 passages.
|
|
|
Embryonic Stem Cell Qualified FBS Performance Compared to Alternative Supplier.
Three mouse embryonic stem cell (ESC) lines (D3, V6.5, and E14) were cultured for 4 passages in media supplement with 15% FBS from either R&D Systems or an alternative commercial supplier. A) Representative brightfield images of D3 mouse ESC colonies cultured in R&D Systems FBS or Supplier A. Normal colony formation was observed for both serum conditions. B) Quantitative analysis of ESC expansion following 4 passages indicates R&D Systems FBS performs similar to an alternative supplier of FBS.
|
Preparation & Storage
| Shipping Conditions |
On Dry Ice
|
| Storage |
-5 to -20 °C
|
Customer Reviews for Fetal Bovine Serum - Embryonic Stem Cell Qualified
Have you used Fetal Bovine Serum - Embryonic Stem Cell Qualified?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
FAQs for Fetal Bovine Serum - Embryonic Stem Cell Qualified
Product Documents for Fetal Bovine Serum - Embryonic Stem Cell Qualified
|