Breadcrumb
- Home
- Products
- Mesenchymal Stem Cells
- Mesenchymal Stem Cells Flow Cytometry Kits
- Human Mesenchymal Stem Cell Multi-Color Flow Kit (FMC002)
Human Mesenchymal Stem Cell Multi-Color Flow Kit
R&D Systems, part of Bio-Techne | Catalog # FMC002

Key Product Details
Summary for Human Mesenchymal Stem Cell Multi-Color Flow Kit
Kit Summary
For verification of mesenchymal stem/stromal cell identity by flow cytometry.
Key Benefits
- Verifies MSC identity in less than 2 hours
- Conjugated antibodies allow for single step staining
- Defines the starting cell population
- Multiple markers reduce experimental variation
Why is it important to verify MSC identity?
Human mesenchymal stem/stromal cells (MSCs) can be isolated by a variety of techniques, grown and expanded using numerous reagents, and differentiated with a range of media, proteins, and small molecules.
Variations in techniques as well as differences in the MSC starting population may account for experimental variability and some of the contradictory data in the literature. By clearly defining the starting cell populations, researchers can decrease experimental variability and ultimately decrease apparent discrepancies in the literature that stem from the use of phenotypically different cell populations. R&D System offers the Human Mesenchymal Stem Cell Multi-Color Flow Cytometry Kit which is used to define human MSCs based on established markers. The Human MSC Multi-color Flow Cytometry Kit includes four fluorochrome-conjugated antibodies for the detection of MSC markers by flow cytometry.
The Human Mesenchymal Stromal Cell Multi-Color Flow Cytometry Kit:
- Efficiently verifies MSC identity using flow cytometry.
- Defines the starting cell population to reduce experimental variability.
- Includes conjugated-antibodies to reduce hands-on time.
- Includes multiple MSC markers to increase confidence in MSC identity.
Mesenchymal Stromal Cells or Mesenchymal Stem Cells?
The term ‘mesenchymal stromal cells’ is commonly used to describe a heterogeneous population of cultured cells that are adherent to plastic, have a distinct morphology, and express a specific set of marker proteins. Within this heterogeneous population are cells referred to as ‘mesenchymal stem cells.’
Mesenchymal stem cells are multipotent, self-renewing cells that have the ability to differentiate into adipocytes, chondrocytes, and osteoblasts when cultured in vitro.
2006 Proposed Change to MSC Nomenclature
Although mesenchymal stromal cells were once referred to as ‘mesenchymal stem cells,’ a change to ‘mesenchymal stromal cells’ was proposed by the International Society for Cellular Therapy in 2006.1
The change in nomenclature originates from two important factors:
- Methods used to isolate mesenchymal stem cells yield a heterogeneous population of cells with only a fraction of these cells demonstrating multipotency.
- The absence of direct evidence that mesenchymal stem cells can self-renew and differentiate in vivo.
Use of Mesenchymal Stem and Stromal Cell Terminology
Data supporting MSC self-renewal and multipotency have been obtained using in vitro conditions, which does not adequately reflect the in vivo environment. The lack of in vivo data has led some researchers to question the validity of the term ‘mesenchymal stem cell’ providing further support for the use of ‘mesenchymal stromal cells’ to describe MSCs.2 While ‘mesenchymal stromal cells’ may be the more scientifically accurate term for MSCs, the two terms are often used interchangeably in the literature. R&D Systems recognizes the use of both mesenchymal stem cells and mesenchymal stromal cells and uses ‘MSC’ to indicate mesenchymal stem/stromal cells to account for both designations.
Definitions of Mesenchymal Stromal Cells and Mesenchymal Stem Cells
- Mesenchymal Stromal Cells – A heterogeneous population of cultured cells with similar characteristics such as the ability to adhere to plastic and the expression of specific marker proteins.
- Mesenchymal Stem Cells – A subpopulation of mesenchymal stromal cells that have the capacity to self-renew and differentiate into mesodermal lineages when cultured in vitro. The capacity to self-renew and differentiate in vivo has yet to be clearly demonstrated for mesenchymal stem cells.
References
- Dominici, M. et al. (2006) Cytotherapy 8:315.
- Keating, A. (2012) Cell Stem Cell 10:709.
Kit Components
The Human Mesenchymal Stromal Cell Multi-Color Flow Cytometry Kit contains four conjugated monoclonal antibodies and corresponding isotype controls that can be used for single-step staining of human mesenchymal stem/stromal cells. This kit includes sufficient reagents for 25 assays. See Details
- Flow Cytometry Staining Buffer (100 mL)
Positive Markers
- Mouse IgG1 Anti-Human CD105-PerCP (Clone 166707)
- Mouse IgG1 Anti-Human CD146-CFS (Clone 128018)
- Mouse IgG2A Anti-Human CD90-APC (Clone Thy-1A1)
Negative Markers
- Mouse IgG1 Anti-Human CD45-PE (Clone 2D1)
Stability and Storage
Store at 2 °C to 8 °C in the dark. Use within 6 months of receipt.
Precautions
The Staining Buffer contains 0.1% sodium azide. Sodium azide may react with lead and copper plumbing to form explosive metallic azides. Flush with large volumes of water during disposal.
The term 'mesenchymal stem cells' (MSCs) is most commonly used to describe multipotent self-renewing cells that can be differentiated in vitro to generate adipocytes, chondrocytes, and osteoblasts. However, because these biological properties and hierarchical relationships remain to be clearly demonstrated in vivo, the term 'multipotent mesenchymal stromal cells' is often used to distinguish cultured cells from their in vivo precursors. Originally discovered in mouse bone marrow, multipotent mesenchymal stromal cells cultured from a variety of species and tissue types, have been shown to differentiate into progeny of additional lineages including, cardiomyocytes, endothelial cells, hepatocytes, and neural cells. Again, the physiological relevance of these findings remains to be determined.
| Species | Human |
| Source | N/A |
|
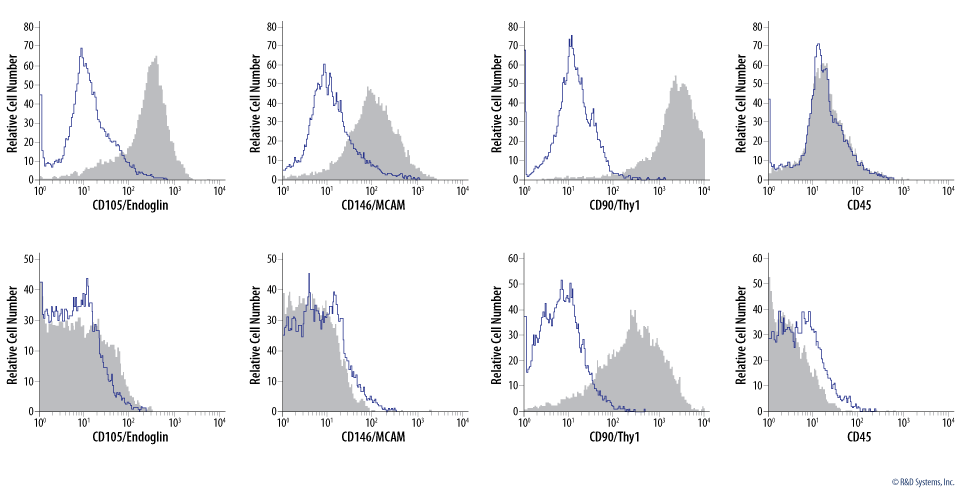
Verification of Mesenchymal Stem/Stromal Cell Identity by Analysis of Mesenchymal Stem/Stromal Cell Marker Expression. Undifferentiated (A) and osteocyte-differentiated (B) human mesenchymal stem cells were stained with the indicated antibodies (filled histograms) or the corresponding isotype control (open histograms), using the Human Mesenchymal Stromal Cell Multi-Color Flow Cytometry Kit (Catalog # FMC002). Multi-color flow cytometry simultaneously detected cells that were positive for MSC markers (CD105/Endoglin, CD146/MCAM, and CD90/Thy1). Following 21 days of osteocyte-differentiation, the cells showed a characteristic reduction in CD105/Endoglin, CD146/MCAM, and CD90/Thy1 staining. CD45 is a negative control for both cell types. |
Preparation & Storage
| Shipping Conditions | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Storage | Store the unopened product at 2 - 8 °C. Do not use past expiration date. |
Assay Procedure
Refer to the product datasheet for complete product details.
Briefly, the starting population of cells is characterized for MSC markers using the following procedure:
- Incubate the cells with the provided antibodies
- Wash the cells
- Resuspend in Flow Cytometry Staining Buffer
- Analyze by flow cytometry
Reagents Provided
Reagents Supplied in the Human Mesenchymal Stromal Cell Multi-Color Flow Cytometry Kit (Catalog # FMC002):
- Flow Cytometry Staining Buffer (100 mL)
Positive Markers
- Mouse IgG1 Anti-Human CD105-PerCP (Clone 166707)
- Mouse IgG1 Anti-Human CD146-CFS (Clone 128018)
- Mouse IgG2A Anti-Human CD90-APC (Clone Thy-1A1)
Negative Markers
- Mouse IgG1 Anti-Human CD45-PE (Clone 2D1)
Other Supplies Required
Reagents
- Fc Receptor Blocking Reagents
Materials
- Flow Cytometry/FACS™ Tubes (5 mL round-bottom polystyrene tubes)
- Pipette Tips and Pipettes
Equipment
- Centrifuge
- Vortex
Procedure Overview
This protocol has been tested using bone marrow- and/or adipose tissue-derived MSCs. If using a different tissue source or cell line, the protocol below may need to be optimized.






- Wash the cells with Flow Cytometry Staining Buffer.
Perform a cell count. - Add Fc receptor blocking reagents. If using excess pre-immune IgG to block Fc receptor, the excess IgG does not need to be washed from the cells following the incubation period.
- Transfer approximately 100 µL of the Fc receptor-blocked cells (about 1 x 106 cells) into a 5 mL flow cytometry tube.
- Add 10 µL of each fluorochrome-conjugated antibody or each corresponding isotype control antibody.
- Incubate the mixture for 30-45 minutes at room temperature in the dark.
- Centrifuge the samples at 300 x g for 5 minutes.
- Wash the cells with 2 mL Flow Cytometry Staining Buffer.
- Resuspend the cell pellet in 200-400 µL of Flow Cytometry Staining Buffer.
- Analyze expression of MSC markers simultaneously by flow cytometry.
