- Tandem dye antibody conjugates
- Forster- or fluorescence-resonance energy transfer (FRET)
- FRET dyes or FRET pairs
- Stroke shift effect
- Photobleaching of tandem dyes
- Degradation of tandem dyes
- Compensation controls
- What are tandem dyes?
- How do tandem dyes work?
- What steps can be taken to avoid degradation of tandem dyes?
- Are tandem dyes sensitive to fixatives and permeabilization agents?
- What should you consider when preparing compensation controls for tandem dyes in flow cytometry?
- Why are tandem dyes not recommended for intracellular staining?
What are tandem dyes?
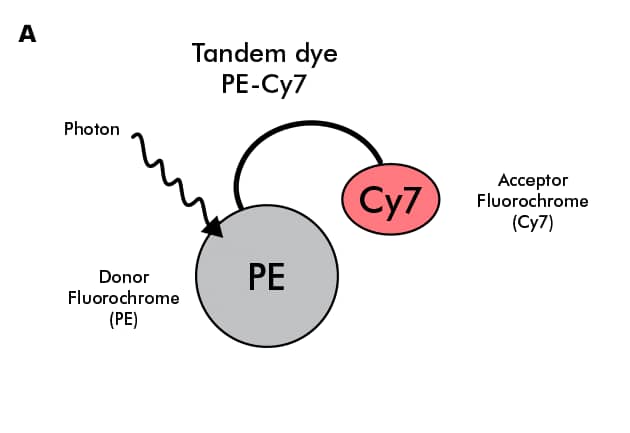
A tandem dye, also called a FRET pair or FRET dye, is a pair of covalently linked fluorescent molecules (fluorochromes or fluorophores) which contain a donor and an acceptor molecule (Figure 1A). A donor molecule is generally either a protein-based dye [e.g. Phycoerythrin (PE) ] or a chemically synthesized dye with a large extinction coefficient (i.e. capacity to absorb energy in the form of light/photons). An acceptor molecule is a synthetic dye [e.g. Cyanine-7 (Cy7)] which absorbs energy emitted by the donor molecule through a phenomenon called Forster- or fluorescence-resonance energy transfer (FRET) (Beavis & Pennline, 1996, Forman & Gupta, 2007). Examples of tandem dyes include PE-Cy7, PE-Cy5.5, PE-Atto 594, and APC-Cy7.
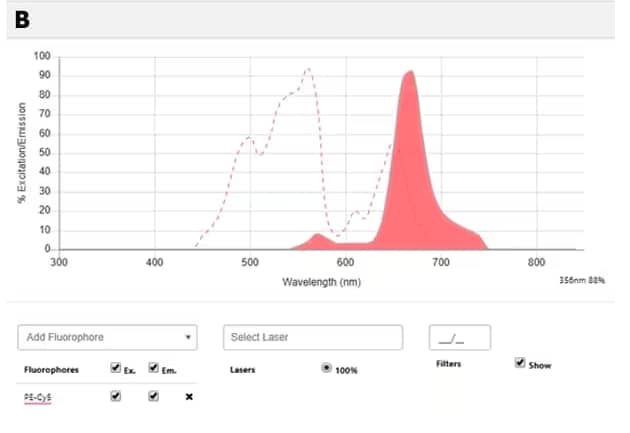
Figure 1: Tandem dyes A. Diagrammatic representation of transfer of energy between donor and acceptor molecules in a tandem dye PE-Cy7. B. Spectral image of PE-Cy7 tandem dye showing the excitation spectrum (dotted line) and the emission spectrum (shaded area with solid line). Try our Spectra Viewer to explore the excitation and emission spectra of various tandem and non-tandem dyes.
|
How do tandem dyes work? |
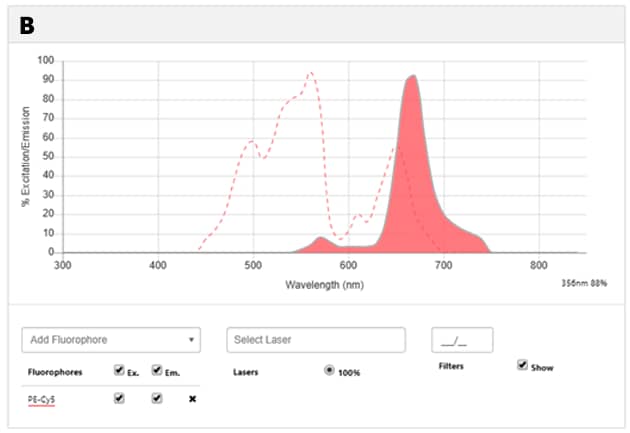
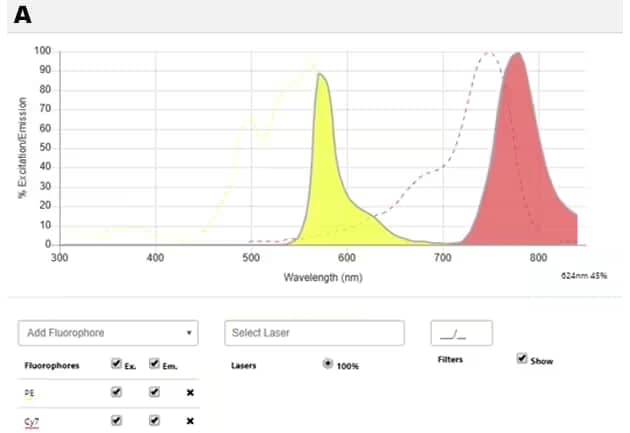
Figure 2: Spectral properties of PE, Cy7 and PE-Cy7 dyes: A. Spectral diagram of excitation and emission spectra of PE (yellow) and Cy7 (red). B. Spectral diagram of PE-Cy7 tandem dye showing the excitation spectrum (dotted line) and the emission spectrum (shaded area with solid line). NOTE: PE has three absorption maxima (498nm, 544nm, 566nm) and all of them can be used (the optimal one will depend on the application). Suggested excitation laser lines for PE are 488, 532 and 561, and its maximum fluorescence emission is at 700nm. Explore the spectral diagrams of fluorochromes of your choice using our Spectra Viewer
The light emitted by a fluorochrome is of a longer wavelength than what was absorbed during excitation. This difference between the maximum absorbance and emission wavelengths is defined by the Stokes shift. A larger Stokes shift means there is less overlap between the two wavelengths i.e. absorption and emission maxima. Tandem dyes broaden the spectral range detected using single-laser excitation and hence, expand the number of parameters that can be analyzed in multicolor flow cytometry experiments. For example, the 488nm laser can be used to simultaneously excite DyLight® 488, PE, and Peridinin Chlorophyll Protein (PerCP)-Cy5.5 which then generate green, yellow and red emissions respectively.
What steps can be taken to avoid degradation of tandem dyes?
One major limitation of tandem dyes is their sensitivity to degradation. Uncoupling or detachment of donor and acceptor via degradation leads to a loss of emission from the acceptor molecule and an increased emission of the donor molecule. This issue becomes more critical when an experiment involves fluorochromes which are excited by the same laser. For example, if PE-Cy7 is degraded in an experiment which also uses PE, the fluorescence of degraded PE pool from PE-Cy7 will also be detected in the PE channel and this will contribute to artifacts in data analysis. Degradation of tandem dyes depends on several, often avoidable, factors which are described below:
- Photodegradation or Photobleaching: Repeated illumination of fluorochromes in tandem dyes, and/or exposure to direct light during storage or experimentation can break a covalent bond between the two dyes in the tandem pair. Therefore, it is highly critical to protect tandem dye antibody conjugates from exposure to light during storage and when in use. Storing the tandem dye conjugates in dark bottles has been shown to increase the durability or shelf life of tandem pairs (Forman and Gupta, 2007).
- Storage Condition: Tandem dyes antibody conjugates should NEVER be stored at -20°C or other freezing temperatures. Freezing causes denaturation of the donor fluorochrome in a tandem dye and a loss of fluorescence signal. For appropriate storage conditions, it is recommended to double-check the information provided on the product datasheet.
- Sample Incubation: Some tandem dye molecules (e.g. APC) are susceptible to cell-mediated uncoupling of the acceptor and the donor molecules. Therefore, when working with un-fixed cells, it is highly advisable to perform conjugated antibody - cell sample incubation at 4°C or by placing the cell culture plate on top of crushed ice in a bucket. Low temperature slows down cell metabolism which ultimately helps preserve the intra-assay stability of the tandem dye.
- Shelf Life: Tandem dyes and tandem conjugated antibodies are relatively less stable when compared to their corresponding individual dye components. We highly suggest you review the product datasheets for information on recommended storage conditions and the guarantee statement (under Limitations section)
Are tandem dyes sensitive to fixatives and permeabilization agents?
Yes, fixation (fixatives) and permeabilization (permeabilizing agents such as detergents) can lead to the degradation of tandem dyes. If fixation/permeabilization steps are absolutely required in your assay, their timing should be kept as short and as mild as possible.
What should you consider when preparing compensation controls for tandem dyes in flow cytometry?
For compensation, a single stained sample is needed for each fluorochrome in a multicolor flow cytometry experiment. The compensation control should be an exact match to the fluorochrome, sample type, and experimental condition. Due to significant differences in tandem dye chemistry, the tandem conjugated antibody used in the experiment should be the same (from the same tube) as the one used for compensation. Use of the exact same tandem dye (fluorochrome) but conjugated to another antibody, or even using a different lot of the same antibody preparation can lead to inaccuracies in compensation calculations.
Why are tandem dyes not recommended for intracellular staining?
Tandem conjugated antibodies have difficulty crossing the cell membrane to reach intracellular target proteins due to their large size. Thus, tandem dye antibody conjugates are not recommended for staining intracellular antigens.
References
- Beavis AJ & Pennline KJ. Allo-7: a new fluorescent tandem dye for use in flow cytometry. Cytometry. 1996; 24:390-395
- Forman MA & Gupta RK. Tandem dyes for flow cytometry: can we overcome quality concerns? MLO Med Lab Obs. 2007 Oct;39(10):24, 26.