
At the Advanced Therapies 2024 meeting in London, John Maher, Chief Scientific Officer at Leucid Bio and Consultant and Senior Lecturer at King's College London, gave a presentation on his academic group's T4 immunotherapy study centered on intratumoral pan ErbB targeted CAR-T for head and neck squamous cell carcinoma. This talk told the story of his armored CAR constructs, their application to solid tumors, and clinical outcomes.
Designing CARs Targeting the Erb Network
In this blog, we recap the T4 immunotherapy studies, employing a CAR designed to target extensively across the entire ErbB receptor network, which is overexpressed in many solid tumors. To enhance T cell expansion and persistence, the T4 CAR incorporates a novel chimeric cytokine receptor that allows the cells to proliferate in response to IL-4, typically a poor growth factor for T cells.
Efficiency can be a real issue. With that in mind, John and his team designed a chimeric cytokine receptor called 4αβ where the ectodomain of the IL-4 receptor is coupled to the shared beta chains of the receptors for IL-2 and IL-15. This enables the use of IL-4, normally a very poor growth factor for T cells, as a selective mitogen for the T cells which have taken up retroviral vector encoding these two transgenes. The elephant in the room in this story is safety. John’s rhetorical question was a poignant one: “how can you ever possibly target the extended Erb network and hope to get away with it from a safety point of view?”
This safety concern was the influencing factor in deciding which indication to target; the final decision being head and neck cancer. These tumors often exhibit locally advanced or recurrent disease, providing a clinical model to assess the safety of this approach, albeit still a risky endeavor. The prognosis of a patient with an untreatable tumor of this type was one of the key arguments made to the regulators before beginning the trial. The trial itself was a pretty standard design for a phase I oncology study; a dose escalation, 3 + 3 design with an initial cohort of patients receiving 10 million CAR-T cells directly into the target lesion, and then escalating up to a maximum deliverable dose of a billion CAR-T cells.
Phase I T4 Immunotherapy Clinical Trial Outcomes
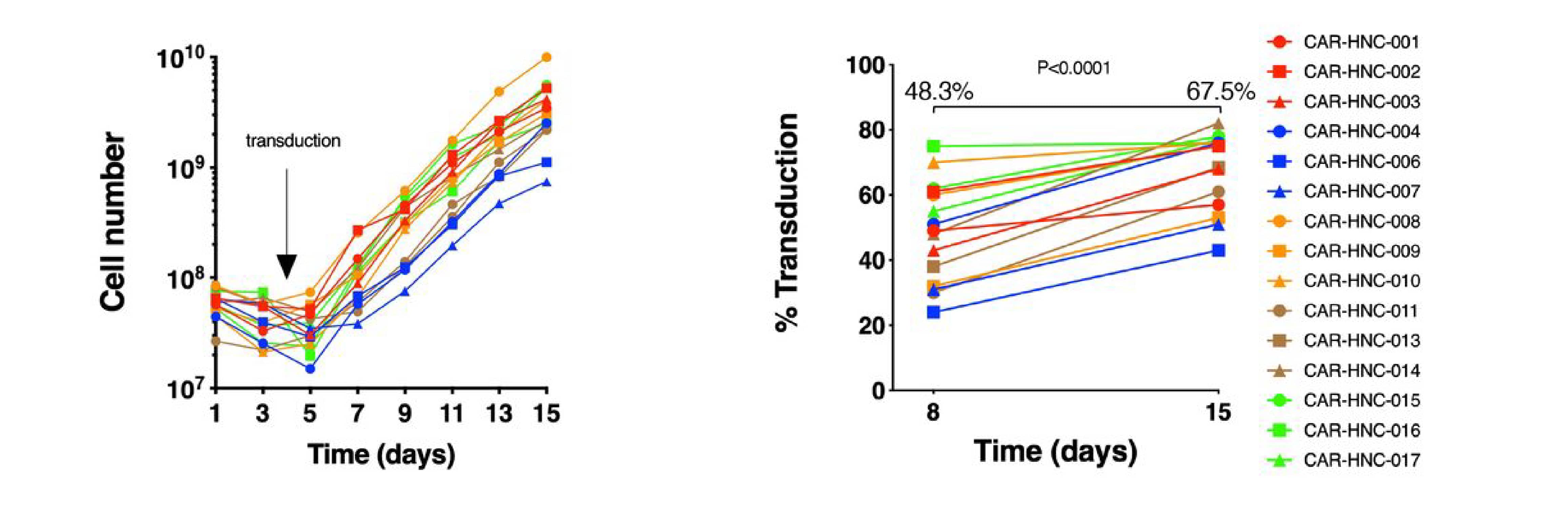
Figure 1: Fold expansion of T4 CAR T cells (left) and proportion of transduced cells measured by flow cytometry at the midpoint and end of the 2-week manufacturing process (right). Image sourced from Papa S, Adami A, Metoudi M, et al. (Journal for ImmunoTherapy of Cancer, 2023)1 licensed under CC BY 4.0.
The 10-14 day vein to tumor manufacturing process is quite different to a standard CAR-T product. The first difference is that the starting material is whole blood, not an apheresis, making it significantly easier to get started. John explained that the aim was to get 120- 130 mL of blood, but in one patient they only managed to get as little as 40 mL, and yet the cells grew well across the board. This reflects the selective expansion of the transduced cells in response to IL-4 and because of that selective enrichment, if you compare the percentage of CAR positive cells at the midpoint and at the end of manufacture, there is an upward inflection due to selection for the desired cells.
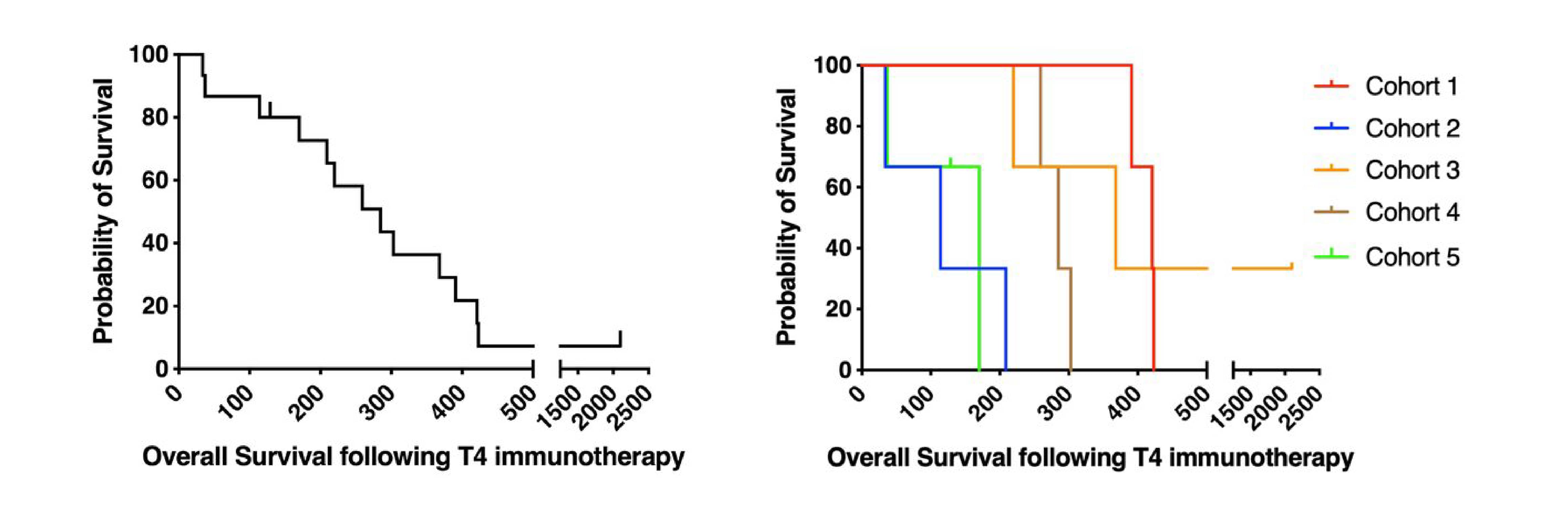
Figure 2: Kaplan-Meier survival curves showing outcomes of all patients (left) and individual patient cohorts (right). Image sourced from Papa S, Adami A, Metoudi M, et al. (Journal for ImmunoTherapy of Cancer, 2023)1 licensed under CC BY 4.0.
Overall, the phase I trial has shown a favorable safety profile, with manageable local inflammation and fever as the primary side effects. From an efficacy point of view, while T4 is not as potent as CD19-targeted CAR-Ts in blood cancers, it did demonstrate signs of clinical activity, with 9 out of 15 patients achieving stable disease. In one case, there was a long-term survivor who achieved stable disease with T4 and then went on to receive an oncolytic virus, T-VEC, in combination with pembrolizumab and he achieved a sustained complete response for over three and a half years, highlighting the potential for combination strategies.
John and his team went back to the CAR-T products to try to understand which patients did better and why. Here are the factors that led to better outcomes:
- A high CD4 to CD8 ratio in the CAR-T product
- Lack of expression of exhaustion markers
- High expression of the glucose transporter GLUT1 on the CD4 cells
- Poly-functionality of these CAR-T cells
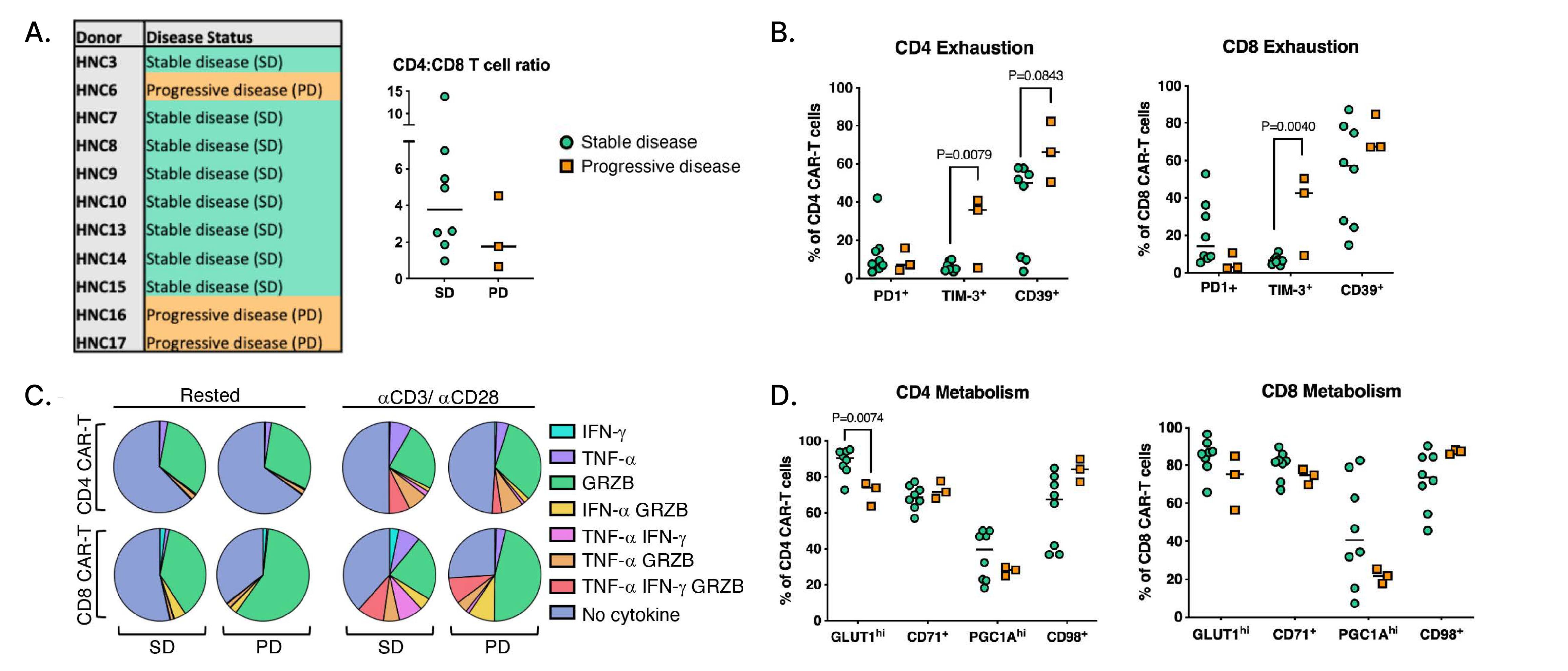
Figure 3: Immunophenotypic analysis of T4 CAR-T cell batches in stable disease (SD) and progressive disease (PD) patients.
A. Outcomes of patients whose pre-infusion CAR-T phenotype was assessed in surplus retention samples and flow cytometry analysis of cryopreserved PBMCs from the infusion product, showing CD4 to CD8 CAR-T cell ratios by disease status.
B. Frequency of PD1+, TIM-3+, and CD39+ CD4+ and CD8+ CAR-T cells.
C. Mean proportion of CD4+ and CD8+ CAR-T cells in SD and PD subjects single, double, or triple positive for Granzyme B (GRZB), IFN-γ, and TNF-α.
D. Representative flow cytometry gating of GRZB+, IFN-γ+, and TNF-α+ CD8+ CAR-T cells in an SD and PD subject with healthy donor T cells as control.
Image sourced from Papa S, Adami A, Metoudi M, et al. (Journal for ImmunoTherapy of Cancer, 2023)1 licensed under CC BY 4.0.
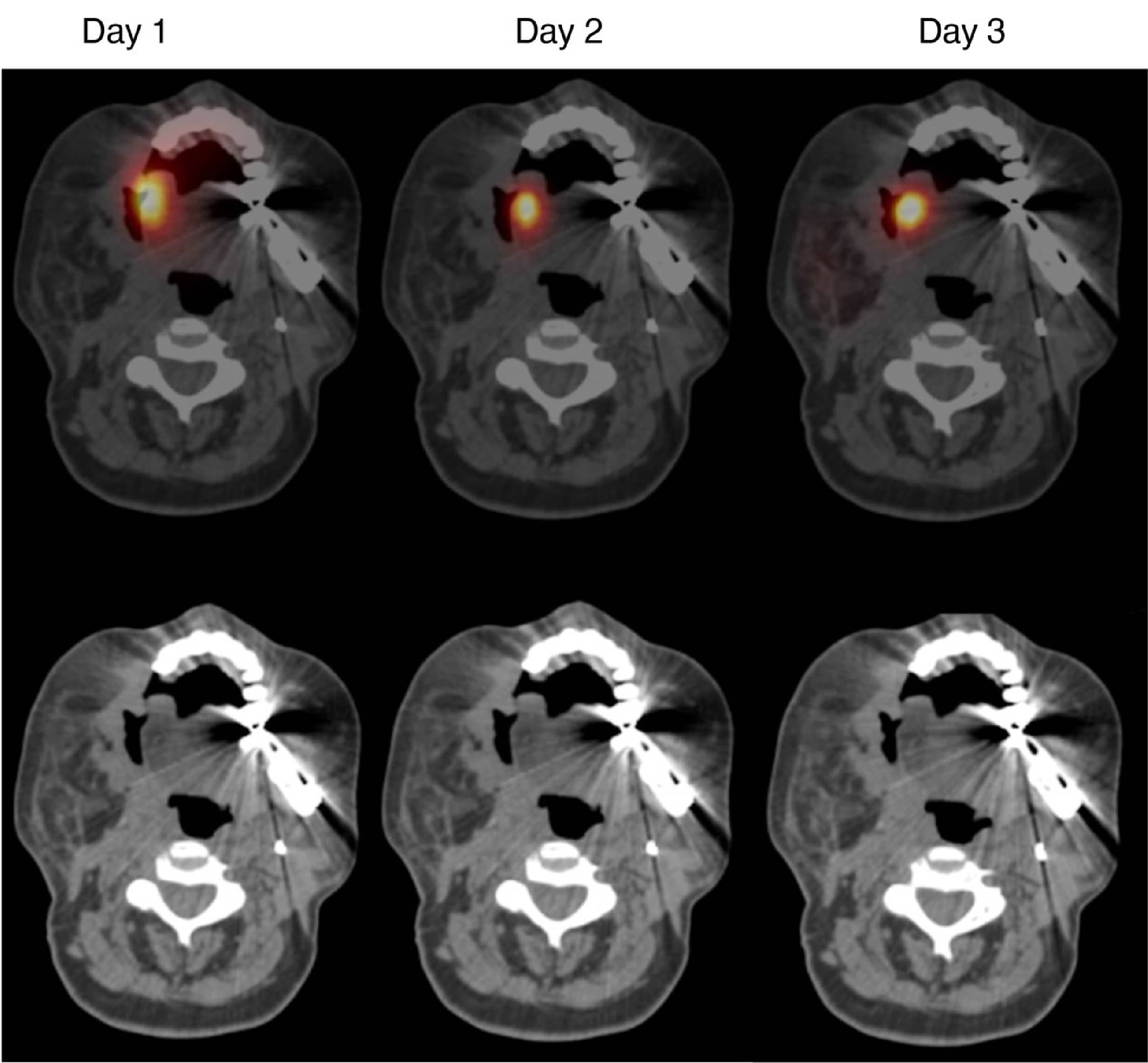
Visualizing Biodistribution of
CAR-T Cells
The final part of the study involved one patient where the team took a small aliquot of the CAR-Ts, labeled them with indium, and put them into the tumor to visualize their biodistribution. The area on the side of the head is all tumor and the tracer sits on top of the target lesion, which John likened to a cherry on top of a cake. When sequential SPECT/CT imaging is performed, even 48 hours later the cells are pretty much where you put them.
This has its pros and cons. It's positive because there is no systemic absorption leading to toxicity but, on the other hand, the cells aren’t infiltrating into the target lesion.
Figure 4: SPECT-CT imaging of radiolabeled T4 CAR T cells. Image sourced from Papa S, Adami A, Metoudi M, et al. (Journal for ImmunoTherapy of Cancer, 2023)1 licensed under CC BY 4.0.
-
Papa S, Adami A, Metoudi M et al. (2023) Intratumoral pan-ErbB targeted CAR-T for head and neck squamous cell carcinoma: interim analysis of the T4 immunotherapy study. Journal for ImmunoTherapy of Cancer
