By Jamshed Arslan Pharm.D.
Alzheimer's disease (AD) is an irreversible brain disorder that destroys memory and thinking skills. The telltale signs of AD brains are extracellular deposits of amyloid beta (Aβ, a polypeptide that comes from proteolysis of precursor protein APP) and intracellular neurofibrillary tangles. The consequent neurodegeneration affects regions associated with memory and cognition, the most noteworthy of which is the hippocampus. It goes without saying that generating immortalized primary hippocampal neurons that could express AD-related protein(s) would immensely facilitate the study of underlying mechanisms of AD. So, a team of scientists from Texas Tech University and Graduate School of Biomedical Sciences, Lubbock, TX, used hippocampal HT22 cells transfected with human mutant APP cDNA (HT22-mAPP) to determine the effects of two AD-related proteins: amyloid beta and mAPP. They found that these proteins induce impaired autophagy and mitophagy, synaptic abnormality, and structural and functional damage to the mitochondria, thereby explaining the neuronal dysfunction in AD.
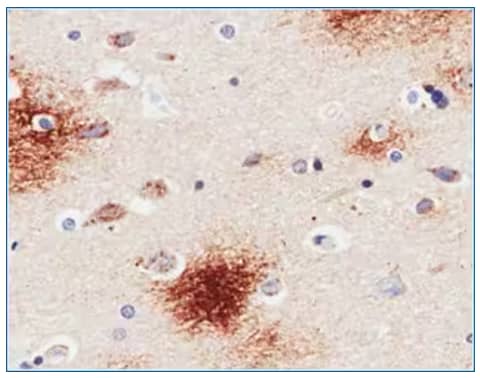
Immunohistochemistry-Paraffin: Amyloid beta Peptides Antibody (6E10) [NBP2-62566]- Immunohistochemical staining of human Alzheimer's disease hippocampus tissue using anti-Amyloid Beta antibody. 6E10 Anti-Amyloid Beta staining of paraffin embedded human hippocampus affected by Alzheimer's disease using the rabbit-chimeric version of 6E10 [NBP2-62566]. Antigen retrieval was achieved by microwaving in citrate buffer (pH6), followed by blocking with protein block serum-free buffer. Primary antibody incubation with NBP2-62566 was carried out at 4 ug/ml for 30 minutes. Samples were then incubated with an anti-rabbit IgG HRP secondary antibody [NBP2-24891] for 20 mins followed by DAB (3,3'-diaminobenzidine), and counter-staining with hematoxylin. Staining of amyloid plaques in the parahippocampal gyrus may be observed. Recommended concentration, 2-4 ug/ml.
mAPP and amyloid beta induce changes in gene and protein expression that are indicative of AD-related neuronal defects
The team first demonstrated an appropriate transfection efficiency in HT22-mAPP cells by showing full-length 110 kDa mAPP and increased amyloid beta 42 levels in them. To study the effects of these two proteins (mAPP and amyloid beta), researchers performed real-time qRT-PCR and immunoblotting and reported a differential expression in transfected cells relative to the untransfected controls. Following are some of the key observations in HT22-mAPP cells that were further corroborated by immunofluorescence analysis:
- Increased mitochondrial fission (increased Drp1 and TTTC11)
- Reduced mitochondrial fusion (decreased Mitofusin 1, Mitofusin 2 and OPA1)
- Decreased mitochondrial biogenesis (reduced PGC1 alpha, Nrf1, Nrf2 and mtTFA)
- Lowered synaptic functions (diminished synaptophysin, PSD-95, and MAP2)
- Diminished autophagy (lowered expression of ATG5, Beclin 1, LC3A, and LC3B)
- Reduced mitophagy (decreased PINK1, TERT, Bcl-2 and BNIP3L)
These findings are significant because synaptic and mitochondrial impairments are among the early events in AD pathogenesis. The team further explored mitochondrial functioning in HT22-mAPP cells.
mAPP and amyloid beta reduce mitochondrial function
Researchers found significantly increased levels of hydrogen peroxide and 4-hydroxy-2-nonenol (indicator of lipid peroxidation) and decreased cytochrome C oxidase activity and ATP production in the HT22-mAPP cells relative to the controls. Likewise, transmission electron microscopy indicated increased number of shorter mitochondria, indicating that AD-related proteins fragmented the mitochondria in hippocampal neurons. As expected, the compromised synaptic and mitochondrial functioning resulted in significantly reduced survival of HT22-mAPP cells, which is reminiscent of the compromised neuronal cell survival in advanced AD.
All in all, the deficiencies in synaptic functioning, mitochondrial dynamics, autophagy and mitophagy in hippocampal neurons explain the neuronal dysfunction seen in AD brains.
Significance
This study has vast therapeutic implications. Generating immortalized hippocampal neuronal cells transfected with mAPP cDNA opens the gate for testing potential anti-AD drugs. Likewise, correcting the dysfunctional mitochondrial biogenesis, autophagy and mitophagy in hippocampal neurons, as found in the current study, are considered valid therapeutic approaches in AD.

Jamshed Arslan, Pharm D.
University of Alabama at Birmingham, School of Medicine
Dr. Arslan studies cell signaling in mitochondrial defects in C. elegans
and transgenic mice.
-
Reddy, P. et al. (2018) Mutant APP and Amyloid Beta-Induced Defective Autophagy, Mitophagy, Mitochondrial Structural and Functional Changes and Synaptic Damage in Hippocampal Neurons from Alzheimer's Disease Human Molecular Genetics 27:2502-2516.