By Michalina Hanzel, PhD
In this second installment of our three blog-posts series on major cellular mechanisms responsible for neurodegenerative disorders, we will explore the processes of mitochondrial dysfunction and oxidative stress.
Reactive oxygen species and mitochondrial function in neurons
Neuronal cells are especially dependent on correct mitochondrial functions due to their high metabolic rate, low levels of antioxidants and low regenerative capacity. Oxidative stress is a condition resulting from an imbalance between the generation of harmful reactive oxygen species (ROS) and their detoxification and elimination. Mitochondria are both the main source of cellular ROS and their main scavenger hence aberrant mitochondrial function results in a non-optimal environment for neurons and plays a key role in the pathophysiology of many neurodegenerative diseases.
ROS are a by-product of normal cellular processes and, whilst highly reactive and unstable, are promptly cleared from the cytoplasm by antioxidant enzymes. If this process is disrupted, lipid peroxidation and protein and DNA oxidation can occur, leading to compromised cellular functions. In Alzheimer's disease, various lipid peroxidation products and proteins with oxidative modifications are found to be elevated in the brain. Oxidative damage to the DNA, which leads to double strand breaks and base modifications, is also increased in the brains of Alzheimer's patients. Additionally, antioxidant activity is significantly reduced, resulting in further acceleration in ROS accumulation and damage.
In similar fashion, oxidative stress damage has been reported in postmortem brain tissue from patients with Parkinson’s disease and Huntington's disease. In the case of Parkinson's, dopamine, the neurotransmitter found in the neurons of the substantia nigra that degenerate in the disease, is especially sensitive to auto- and enzymatic oxidation, generating ROS and reactive dopamine quinones. In turn, oxidized dopamine is toxic to several mitochondrial proteins, including ndufa1/complex I, resulting in chronic production of ROS, and eventual loss of dopaminergic neurons.
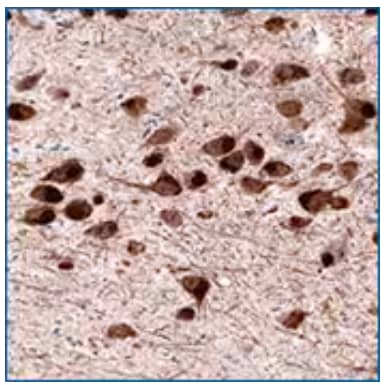
Immunohistochemistry-Paraffin: Dopa Decarboxylase/DDC Antibody (2962) [NBP2-46642] - Staining of human substantia nigra shows cytoplasmic positivity in dopamine neurons in pars compacta.
Mitochondrial dysfunction in neurodegeneration: beyond the generation of reactive oxygen species
Oxidative stress is not the only consequence of mitochondrial dysfunction in neurodegenerative diseases. Mitochondria are also responsible for quality control of proteins transcribed from the nuclear genome that need to be translocated into the organelle from the cytosol through special protein complexes. The functioning of these complexes is often compromised in neurodegenerative diseases. Additionally, molecular chaperones survey this import process in order to avoid misfolding or aggregation of the transported proteins and similarly, disruption in their function can lead to eventual neurodegeneration. For example, in Parkinson’s disease, deficit of mitochondrial import has been linked to the accumulation of alpha-synuclein within the mitochondria. Moreover, low levels of mortalin, a crucial chaperone, have been found in the brains of PD patients. Overall, this and additional data from other neurodegenerative disorders suggest that impairments in quality control systems may lead to the accumulation of defective mitochondria leading to synaptic loss and neuronal degeneration.
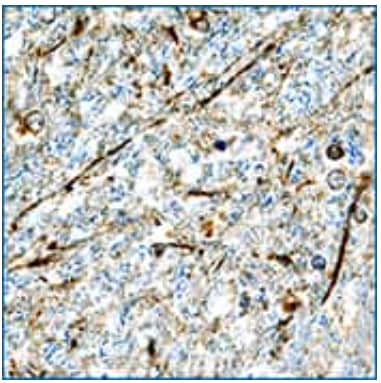
alpha -Synuclein was detected in immersion fixed paraffin-embedded sections of human brain (hypothalamus) using Goat Anti-Human alpha -Synuclein Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1338) at 15 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS008) and counterstained with hematoxylin (blue). Specific staining was localized to neuronal processes.
Mitochondrial quality control as a potential therapeutic target in neurodegenerative diseases
Due to their neuroprotective role when well-functioning, mitochondria are a promising target for drug development in neurodegenerative diseases. Currently, no drugs targeting specifically mitochondrial quality control have been tested in clinical trials but it’s a growing subject of investigation. Previous therapies targeting mitochondrial dysfunction have not produced desired effects partly due to late window of intervention. The current research suggests that the role of mitochondria in neurodegeneration is complex and multifaceted but should be a key theme for future research.
Michalina Hanzel, PhD
Postdoctoral Associate at The Rockefeller University
Dr. Hanzel is studying synaptic function in the cerebellum to understand neurodevelopmental disorders and has a background in developmental neurobiology, molecular and cell biology.
-
Franco-Iborra, S. et al. (2018) Mitochondrial quality control in neurodegenerative diseases: Focus on Parkinson’s disease and Huntington’s disease Frontiers in Neuroscience
-
Grünewald, A. et al. (2018) New insights into the complex role of mitochondria in Parkinson’s disease Progress in Neurobiology
-
Ganguly, G. et al. (2017) Proteinopathy, oxidative stress and mitochondrial dysfunction: Cross talk in alzheimer’s disease and parkinson’s disease Drug Design, Development and Therapy
-
Wang, X. et al. (2014) Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease Biochimica et Biophysica Acta - Molecular Basis of Disease