By Jamshed Arslan, Pharm. D., PhD
Protein phosphorylation refers to a reversible post-translational modification in which a protein kinase adds a phosphate group to an amino acid residue of a target protein. Protein phosphorylation, especially tyrosine phosphorylation, is one of the early events in signal transduction in all eukaryotic cells.
Once a cell is lysed, proteases and phosphatases are released that can degrade or modify proteins, thereby affecting their Western blot detection. The following are some tips for improving Western blot analysis of phosphorylated proteins.
1. Keep samples on ice and use pre-chilled buffers
- The equipment or buffers used in sample processing should be kept cold on ice (4oC). Using pre-chilled reagents and equipment whenever possible can slow down dephosphorylation, proteolysis and denaturation.
2. Use phosphatase inhibitors
- Add phosphatase inhibitors to your lysis buffer solution, preferably in the form of a freshly made protease-phosphatase inhibitors cocktail.
3. Store samples in the loading buffer
- After protein quantification, mix your sample with loading buffer to halt phosphatase activity. The sample can then be aliquoted and stored in the freezer or loaded on the gel.
4. Avoid milk as a blocking agent
- Milk contains abundant amounts of a phosphoprotein, casein, which can cause high background. Instead, use bovine serum albumin (BSA) or protein-free blocking agents.
5. Use phosphate-free buffers
- To minimize nonspecific signals, use TBST instead of phosphate-buffered saline (PBS). If PBS has to be used during intermediate steps, wash the membrane with copious amounts of TBST to remove excess sodium phosphate before adding protein detection substrates.
6. Use sensitive substrates for chemiluminescence detection
- To detect a low abundance protein that is weakly phosphorylated, use antibody to immunoprecipitate the protein in order to concentrate and load more protein in the sample lane. Use highly sensitive substrates for chemiluminescence detection.
7. Detect total protein content
- To figure out if the absence of signal on the Western blot is due to inefficient phosphorylation or inadequate isolation of phosphorylated proteins, probe for total protein content as well. Use a control in which phosphorylated and unmodified forms of proteins are detected, for example, by using paired antibodies.
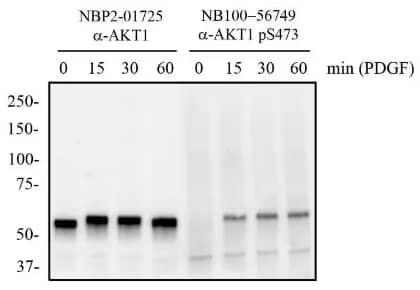
Independent Antibodies Validation and Biological Strategies Validation. AKT1 [p Ser473] Antibody (104A282) (Catalog # NB100-56749) - Mouse 3T3 cells were treated with and without PDGF (50 ng/mL) for 0 to 60 minutes. Total protein from whole-cell lysates was separated on a 7.5% gel by SDS-PAGE, transferred to PVDF and blocked in 5% non-fat milk in TBST. The membrane was probed with 2 μg/mL anti-AKT1 (NBP2-01725) and 2 μg/mL pS473 AKT1 in 1% BSA/ TBST and with an anti-mouse HRP secondary antibody. Signal was visualized with chemiluminescence detection.
To quantify a phosphorylated protein, fluorescent conjugated antibodies can be used to detect phosphorylated and total proteins on the same blot in a process called multiplexing. Fluorescent tagged secondary antibodies generated in two different species can show one color for the phosphorylated form and another for the total protein.
In conclusion, WB of phosphorylated proteins is essentially similar to the non-phosphorylated ones, but caution must be exercised to avoid dephosphorylation during sample preparation. The above-mentioned tips are easy to follow and can significantly improve phosphorylated protein detection through Western blot.
NOTE: Western blots can be challenging and time-consuming to perform. When sample volume is limited or there is a large workload, the fully automated Simple Western™ platform from Bio-Techne brand ProteinSimple, is a hands-off approach to achieve reproducible results in less time.

Jamshed Arslan, Pharm D, PhD
Dr Arslan is an Assistant Professor at Dow University of Health Sciences, Pakistan, where he teaches Pharmacology to future pharmacists.
-
Bass, J. J. et al. (2017) An overview of technical considerations for Western blotting applications to physiological research Scandinavian Journal of Medicine and Science in Sports
-
McDonough, A. A. et al. (2015) Considerations when quantitating protein abundance by immunoblot American Journal of Physiology - Cell Physiology
-
Murphy, R. M., & Lamb, G. D. (2013) Important considerations for protein analyses using antibody based techniques: Down-sizing Western blotting up-sizes outcomes. Journal of Physiology
-
Sawasdikosol, S. (2010) Detecting tyrosine-phosphorylated proteins by western blot analysis Current Protocols in Immunology