RNAscope™ Assay for Adherent Cells Cultured on Coverslips
Introduction
This Technical Note provides guidelines to collect and pretreat adherent cells cultured on coverslips for the RNAscope™ Assay. To pretreat the cells, use RNAscope™ Protease III (available in the RNAscope™ Protease III and Protease IV Reagents, Cat. No. 322340 or RNAscope™.
Universal Pretreatment Kit Cat No 322380). Read the Safety Data Sheet (SDS) available on the website, and follow handling instructions. Wear appropriate protective eyewear, clothing, and gloves. For the latest services and support information, contact our Technical Services.
Workflow
Part 1: Materials Required
Materials Provided from ACD
| Reagent | Storage |
| RNAscope™ Target Probes | 2–8°C |
RNAscope™ Universal Pretreatment Kit | Room temp (20–25°C) |
| RNAscope™ Detection Kit | 2–8°C |
| Washing Buffer (50X) | Room temp (20–25°C) |
User-supplied Materials
| Reagent | Supplier | Cat. No. |
| Poly-L-lysine | Sigma | P8920 |
| Sterile distilled water | — | — |
| 100% ethanol (EtOH) | Sigma-Aldrich | 459844-4L |
10X PBS (Mg2+ and Ca2+ free) | Bio Rad | 161-0780 |
| 4% PFA or 10% NBF | — | — |
| SuperFrost® Plus slides | Fisher | 12-550-15 |
| ImmEdge™ Hydrophobic Pen | Vector Labs | H4000 |
Coplin jar or Tissue-Tek® Staining Dishes | — | — |
Cover glass, rectangle, No. 1, 22 x 50 mm | Fisher | 12-545E |
Cover glass, circle, No. 1 12 mm | Fisher | 12-545-80 |
| Tween 20 | Sigma | P5927 |
| Mounting medium | — | — |
Part 2: Sample Preparation
Prepare Cover Glasses
NOTE: Use sterile cell culture reagents and techniques.
- Place the desired number of cover glasses into a sterile 10 cm cell culture dish.
- Dilute poly-L-lysine to a final concentration of 0.01% in 15 mL of sterile water.
- Completely submerge the cover glasses in the diluted poly-L-lysine at ROOM TEMPERATURE (RT) for 15 MIN.
- Aspirate the poly-L-lysine from the dish. Rinse the cover glasses with 25 mL of 1X PBS three times.
- Remove the final 1X PBS rinse. Sterilize the cover glasses by incubating them in 25 mL of 100% ethanol at RT for 5 MIN.
- Remove the ethanol. Allow the cover glasses to air dry.
Cell Culture
- Place 100 µl of a cell suspension containing 1x10 cells/mL, or enough cells to be 50–75% confluent the following day, directly onto a prepared cover glass placed in the proper cell culture vessel.
- Allow cells to settle at least 10 MIN.
- Add the proper amount of cell culture media needed for your container. For example, 10 mL is an appropriate volume for a 10 cm dish.
- Incubate the cells overnight using the recommended growth conditions for your cell type.
NOTE: You will need to determine the exact amount of time needed for different cell types to attach. Cells will dry out if allowed to sit for too long.
Cell Fixation
- Prepare fresh 4% PFA or 10% NBF.
- Remove cell culture media from each cover glass. Gently rinse the cover glasses by submerging them in 1X PBS.
- Remove the 1X PBS. Submerge the cover glasses in 4% PFA or 10% NBF at RT for 30 MIN.
- Remove the fixation solution. Rinse the cover glasses three times by submerging them in 1X PBS.
Dehydrate and Store Cells
- Remove the 1X PBS wash. Submerge the cover glass in 50% ethanol at RT for 1 MIN.
- Remove the 50% ethanol. Submerge the cover glass in 70% ethanol at RT for 1 MIN.
- Remove the 70% ethanol. Submerge the cover glass in 100% ethanol at RT for 1 MIN.
- Replace the 100% ethanol with fresh 100% ethanol.
NOTE: Cover glasses can be stored in 100% ethanol at –20ºC for up to 6 MONTHS.
Part 3: RNAscope™ Assay
Create a Hydrophobic Barrier
- Remove the cover glasses from the ethanol, and let air dry.
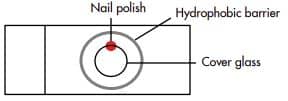
- Immobilize each cover glass on a glass slide by placing a very small drop of nail polish on the slide, then placing the upper edge of the cover glass cell- side up on the nail polish. Press down gently on the cover glass, and allow the polish to dry for 5 MIN.
- Draw a circle around each cover glass using the Immedge™ hydrophobic barrier pen. Let the barrier dry for 5 MIN.
Rehydrate and Permeabilize Cells
- Submerge the slides in a Tissue-Tek®container with fresh 100% ethanol. Incubate at RT for 1 MIN.
- Replace the 100% ethanol with fresh 70% ethanol. Incubate at RT for 1 MIN.
- Replace the 70% ethanol with fresh 50% ethanol. Incubate at RT for 1 MIN.
- Replace the 50% ethanol with 1X PBS. Incubate at
- RT for 1 MIN.
- Replace the 1X PBS with 50 mL of 1X PBS + 0.1% Tween 20 (PBS/T). Incubate at RT for 10 MIN.
- Replace the PBS/T with 1X PBS.
- Replace the 1X PBS with fresh 1X PBS. Rinse the slides by agitating them, and moving them up and down at RT for 1 MIN.
Apply RNAscope™ Hydrogen Peroxide
- Remove excess liquid from the slides, and add 1–3 drops of hydrogen peroxide to each slide. Incubate at RT for 10 MIN.
- Remove the solution, and rinse the slides with 1X PBS twice.
Apply RNAscope™ Protease III
- One at a time, remove each slide from the 1X PBS and tap and or flick to remove excess liquid.
- Place the slides on the HybEZ™ Slide Rack and place rack in the Humidity Control Tray.
- Add 2–4 drops diluted Protease III to completely cover each well/circle.
- Close the Humidity Control Tray and incubate at RT for 10 MIN.
- One at a time, take each slide from the HybEZ™ Slide Rack and tap/flick to remove excess liquid. Submerge slides in 1X PBS.
- Wash the slides by agitating them in the 1X PBS. Repeat with fresh 1X PBS.
NOTE: For most cell lines, dilute protease 1:15 with 1X PBS. Protease dilution factor must be empirically determined for each new cell type.
Probe Hybridization and Staining
Proceed with the RNAscope™ assay steps from target probe hybridization to counterstaining using the appropriate Part 2 Detection User Manual available in the document download center on the product page.
Obtaining Support
For the latest services and support information, contact our Technical Services.
At the website, you can:
- Access telephone and fax numbers to contact Technical Support and Sales
- Search through FAQs
- Submit a question directly to Technical Services