Accelerate the Development of CRISPR-Edited Cell Line Models Using Bio-Techne Single Cell Dispensers
"Utilizing this machine (the Bio-Techne Single Cell Dispenser) has proven instrumental in streamlining the development process of single-cell clones for the cell lines I am working on. It has greatly facilitated the generation of valuable research tools, contributing to the overall progress and efficiency of my work."
- Dr. Oscar Perez-Leal, MD, Assistant Professor, Temple University, School of Pharmacy
CRISPR genome editing brings new frontier in developing better cellular models for drug discovery.
The advent of CRISPR has been revolutionizing how cellular models are created to improve drug discovery. Dr. Perez’s work focuses on utilizing CRISPR genome editing to modify genes for developing cellular models for drug discovery. The approach combines CRISPR and the technology termed FAST-HDR that Dr. Perez and his team developed to knock-in genes or fluorescent tags to potential drug targets or genes encoding proteins that define specific subcellular structures. Once the cells are fluorescently tagged, they can serve as models to perform drug discovery via high-content imaging analysis (Figure 1).
A huge advantage of this approach is that it enables more physiologically accurate models, allowing drug changes to be studied on the endogenous targets without artificially inducing a cell to produce a recombinant protein that may or may not be expressed in the cells normally. This also eliminates tedious cell preparation of fixing and staining the cells to visualize protein expression and localization, and facilitates live-cell analysis.
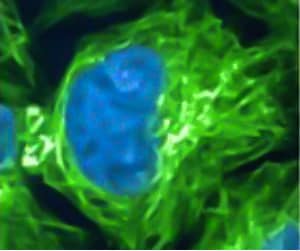
Figure 1. Representative confocal microscopy image of live CRISPR-edited HeLa cells with fluorescent tagging of histone H1 (blue) and β-tubulin (green). Image adapted from the Biomolecules publication by Dr. Perez’s group1.
It is crucial yet challenging to obtain pure clonal population of cells when developing CRISPR-edited cell lines.
Building any cell line requires ensuring all cells are derived from a single parent cell with homogeneous genetic makeup. In Dr. Perez’s experience, obtaining a pure clonal population of CRISPR-edited cells has been a major challenge.
Dr. Perez’s team had used traditional FACS sorting for single cell isolation for cloning. Apart from the inconvenience of having to prepare the cells in his lab and then move the cells to another building where the FACS core was, he had experienced difficulties to grow cell clones out of sorted cells. Especially with stem cells, Dr. Perez was getting essentially no clonal growth at all.
The Bio-Techne Single Cell Dispenser is a powerful tool that greatly improves single cell cloning workflow and outcome.
The introduction of the Bio-Techne Single Cell Dispenser brought significant improvement to workflow ease-of-use, where the cells are prepared and sorted in the same room. On average, Dr. Perez obtains 3-4 times more clonal outgrowth than before. Even with stem cells, he can expect 15 or more clones per 96-well plate. In addition, unlike the routine maintenance required for many other sorting devices, the Bio-Techne instrument requires no maintenance, giving the team peace of mind in between uses. Dr. Perez’s team now has the Bio-Techne Single Cell Dispenser in their standard cell line development and compound library screening workflow, as described in the group’s recent publication1 and summarized in Figure 2.
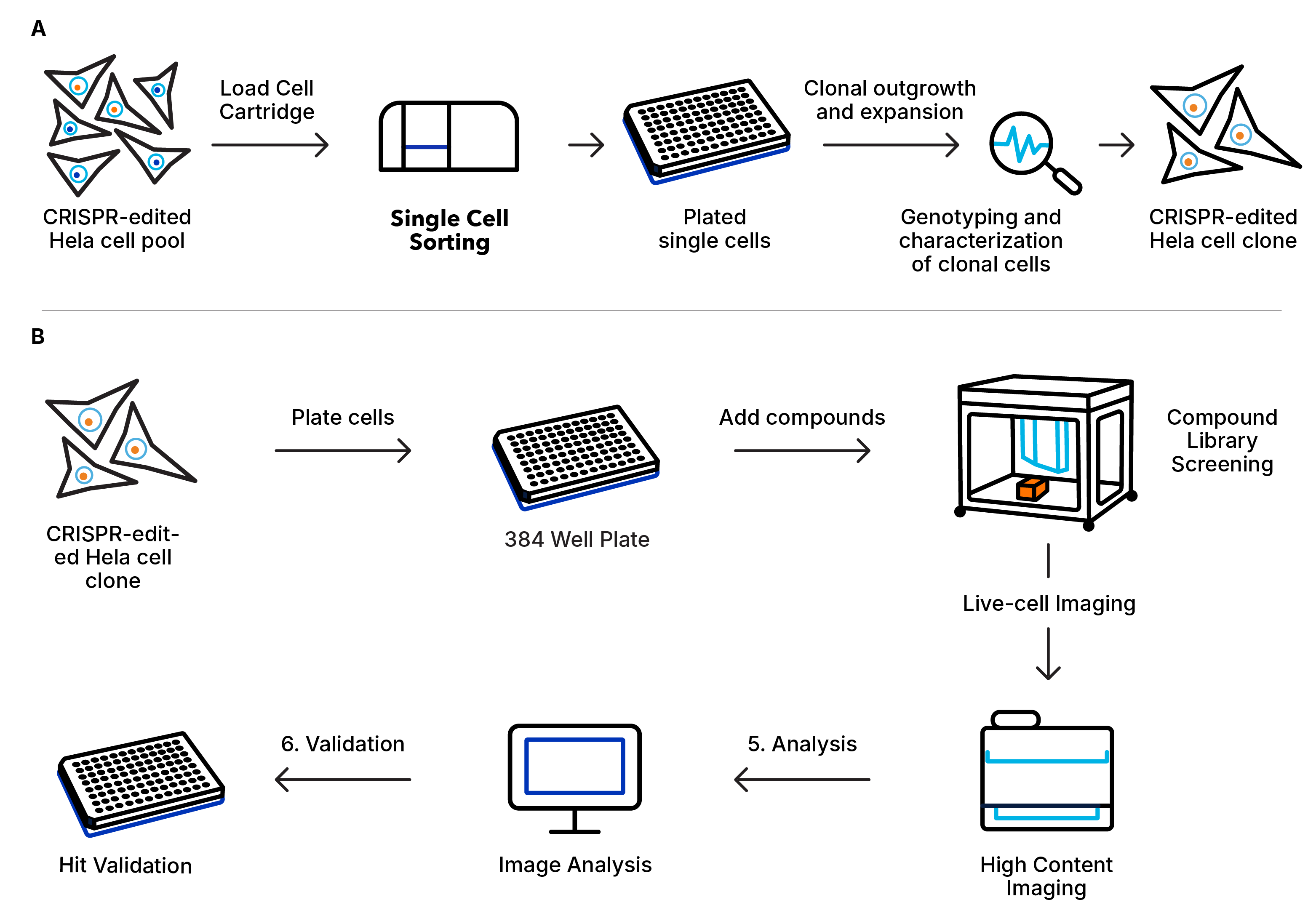
Figure 2. Schematic representation of the steps in the workflows of A) establishing a clonal CRISPR-edited Hela cell line; B) detecting phenotypic changes of CRISPR-edited Hela clonal cell line using high content imaging after treatment with a library of kinase inhibitors. Adapted from the Biomolecules publication by Dr. Perez’s group1.
Selected Publications
-
Khachatryan H, Olszowy B, Barrero C (2023) Identification of Inhibitors of Tubulin Polymerization Using a CRISPR-Edited Cell Line with Endogenous Fluorescent Tagging of β-Tubulin and Histone H1 Biomolecules 13:249. doi: 10.3390/biom13020249. PMID: 36830618; PMCID: PMC9953358. PMID: 36830618.