CD19 Antibody (HIB19) [PerCP/Cy5.5]
Novus Biologicals, part of Bio-Techne | Catalog # NBP1-42856

Conjugate
Catalog #
Forumulation
Catalog #
Key Product Details
Species Reactivity
Human
Applications
Flow Cytometry
Label
PerCP/Cy5.5 (Excitation = 488 nm, Emission = 695 nm)
Antibody Source
Monoclonal Mouse IgG1 kappa Clone # HIB19
Concentration
5 mg/ml
Product Specifications
Immunogen
The immunogen for this antibody was CD19.
Clonality
Monoclonal
Host
Mouse
Isotype
IgG1 kappa
Scientific Data Images for CD19 Antibody (HIB19) [PerCP/Cy5.5]
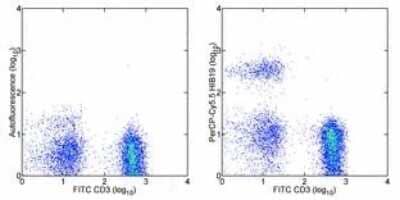
Flow Cytometry: CD19 Antibody (HIB19) [PerCP/Cy5.5] [NBP1-42856] - Staining of normal human peripheral blood cells with Anti-Human CD3 FITC and staining buffer (autofluorescence) (left) or Anti-Human CD19 PerCP-Cy5.5 (right). Cells in the lymphocyte gate were used for analysis.
Applications for CD19 Antibody (HIB19) [PerCP/Cy5.5]
Application
Recommended Usage
Flow Cytometry
1:10-1:1000
Application Notes
This HIB19 antibody has been pre-titrated and tested by flow cytometric analysis of normal human peripheral blood cells. This can be used at 5 uL (0.125 ug) per test. Cell number should be determined empirically but can range from 10^5to 10^8cells/test.
Formulation, Preparation, and Storage
Purification
Protein A or G purified
Formulation
PBS (pH 7.2) with 0.1% gelatin and 0.2% BSA
Preservative
0.09% Sodium Azide
Concentration
5 mg/ml
Shipping
The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below.
Stability & Storage
Store at 4C in the dark.
Background: CD19
Considering the role of CD19 in BCR signaling and its expression in development from pre-B cells through plasma cells, it is understandable that CD19 dysfunction and abnormal expression is associated with numerous B cell malignancies and autoimmune disorders (1-5). CD19 expression is typically observed at relatively normal levels in B cell acute lymphoblastic leukemia (B-ALL) and chronic lymphoblastic leukemia (CLL) but is often reduced other types of lymphoma including diffuse large B cell lymphoma (DLBCL) and follicular lymphoma (FL) (1,2). On the other hand, CD19 expression is typically increased in autoimmune disorders such as systemic sclerosis (SSc) and multiple sclerosis (MS) as modeled by experimental autoimmune encephalomyelitis (EAE) (2). CD19 has become a therapeutic molecular target for the treatment of B cell lymphomas and autoimmune disorders using monoclonal antibodies (mAbs), bi-specific T cell engaging (BiTE) antibodies, and CD19-specific chimeric antigen receptor (CAR) T cells (1,2,4-6). Although anti-CD19 CAR T cell therapy has become the standard for the treatment of B cell malignancies, patients may experience relapse due to resistance mechanisms (6). Strategies to improve efficacy and limit relapse include combination of CAR T cell therapy with immune checkpoint inhibitors like anti-PD-1 (4,6).
References
1. Wang K, Wei G, Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol. 2012;1(1):36. https://doi.org/10.1186/2162-3619-1-36
2. Li X, Ding Y, Zi M, et al. CD19, from bench to bedside. Immunol Lett. 2017;183:86-95. https://doi.org/10.1016/j.imlet.2017.01.010
3. Wentink MWJ, van Zelm MC, van Dongen JJM, Warnatz K, van der Burg M. Deficiencies in the CD19 complex. Clin Immunol. 2018;195:82-87. https://doi.org/10.1016/j.clim.2018.07.017
4. Frigault MJ, Maus MV. State of the art in CAR T cell therapy for CD19+ B cell malignancies. J Clin Invest. 2020;130(4):1586-1594. https://doi.org/10.1172/JCI129208
5. Penack O, Koenecke C. Complications after CD19+ CAR T-Cell Therapy. Cancers (Basel). 2020;12(11):3445. https://doi.org/10.3390/cancers12113445
6. Bouziana S, Bouzianas D. Anti-CD19 CAR-T cells: Digging in the dark side of the golden therapy. Crit Rev Oncol Hematol. 2021;157:103096. https://doi.org/10.1016/j.critrevonc.2020.103096
Alternate Names
CD19, CVID3, Leu-12
Gene Symbol
CD19
Additional CD19 Products
Product Documents for CD19 Antibody (HIB19) [PerCP/Cy5.5]
Product Specific Notices for CD19 Antibody (HIB19) [PerCP/Cy5.5]
This product is for research use only and is not approved for use in humans or in clinical diagnosis. Primary Antibodies are guaranteed for 1 year from date of receipt.
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...