HLA-DR Antibody (L243) - Azide and BSA Free
Novus Biologicals, part of Bio-Techne | Catalog # NBP2-80773

![Western Blot: HLA-DR Antibody (L243)Azide and BSA Free [NBP2-80773] Western Blot: HLA-DR Antibody (L243)Azide and BSA Free [NBP2-80773]](https://resources.bio-techne.com/images/products/HLA-DR-Antibody-L243-Azide-and-BSA-Free-Western-Blot-NBP2-80773-img0001.jpg)
Conjugate
Catalog #
Forumulation
Catalog #
Key Product Details
Species Reactivity
Human, Baboon, Canine, Primate
Applications
Block/Neutralize, CyTOF-ready, Dual RNAscope ISH-IHC, Electron Microscopy, ELISA, Flow (Cell Surface), Flow Cytometry, Functional, Immunocytochemistry/ Immunofluorescence, Immunohistochemistry, Immunohistochemistry-Frozen, Immunohistochemistry-Paraffin, Immunoprecipitation, In vitro assay, Western Blot
Label
Unconjugated
Antibody Source
Monoclonal Mouse IgG2a Kappa Clone # L243
Format
Azide and BSA Free
Concentration
1 mg/ml
Product Specifications
Immunogen
Human lymphoblastoid cell line (RPMI 8866).
Reactivity Notes
Predicted cross-reactivity with Chimpanzee, Baboon, Cynomolgus, Rhesus, Marmoset, Tamarin, Squirrel Monkey
Clonality
Monoclonal
Host
Mouse
Isotype
IgG2a Kappa
Theoretical MW
28 kDa.
Disclaimer note: The observed molecular weight of the protein may vary from the listed predicted molecular weight due to post translational modifications, post translation cleavages, relative charges, and other experimental factors.
Disclaimer note: The observed molecular weight of the protein may vary from the listed predicted molecular weight due to post translational modifications, post translation cleavages, relative charges, and other experimental factors.
Scientific Data Images for HLA-DR Antibody (L243) - Azide and BSA Free
Western Blot: HLA-DR Antibody (L243)Azide and BSA Free [NBP2-80773]
Western Blot: HLA-DR Antibody (L243) - Azide and BSA Free [NBP2-80773] - Analysis of human spleen (A) and human tonsil (B) tissue using HLA-DR antibody at 2 ug/mL. Image from the standard format of this antibody.Immunohistochemistry: HLA-DR Antibody (L243) - Azide and BSA Free [NBP2-80773]
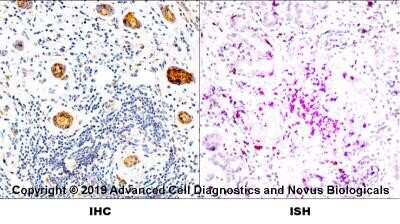
Immunohistochemistry: HLA-DR Antibody (L243) - Azide and BSA Free [NBP2-80773] - Staining of HLA-DR in human spleen using DAB with hematoxylin counterstain. Image from the standard format of this antibody.Flow Cytometry: HLA-DR Antibody (L243) - Azide and BSA Free [NBP2-80773]
Flow Cytometry: HLA-DR Antibody (L243) - Azide and BSA Free [NBP2-80773] - Human peripheral blood lymphocytes stained with L243 PE Image from the standard format of this antibody.Applications for HLA-DR Antibody (L243) - Azide and BSA Free
Application
Recommended Usage
Block/Neutralize
reported in scientific literature (PMID 12874328)
ELISA
reported in scientific literature (PMID 11070170)
Electron Microscopy
reported in scientific literature (PMID 21660937)
Flow Cytometry
0.5 ug/10^6 cells
Functional
reported in scientific literature (PMID 17900279)
Immunocytochemistry/ Immunofluorescence
reported in scientific literature (PMID 7835916)
Immunohistochemistry
1:10 - 1:500
Immunohistochemistry-Frozen
1:10 - 1:500
Immunohistochemistry-Paraffin
1:100
Immunoprecipitation
1:10 - 1:500
In vitro assay
reported in scientific literature (PMID 19620786)
Western Blot
1:100 - 1:2000
Application Notes
It is recommended that the reagent be titrated for optimal performance for each application. This antibody is CyTOF ready.
Formulation, Preparation, and Storage
Purification
Protein A purified
Formulation
PBS
Format
Azide and BSA Free
Preservative
No Preservative
Concentration
1 mg/ml
Shipping
The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below.
Stability & Storage
Store at 4C short term. Aliquot and store at -20C long term. Avoid freeze-thaw cycles.
Background: HLA-DR
Given the role in adaptive immunity, HLA-DR allele polymorphisms, gene misexpression, and dysfunction has been implicated in many diseases ranging from autoimmune disorders to cancer (2). HLA-DR is also a classical biomarker for disease, including sepsis where reduced expression of HLA-DR molecules on monocytes, as measured by flow cytometry, indicates diagnosis and prognosis (4,5). Immunosuppression observed with sepsis results in decreased surface expression of HLA-DR and concurrent increase in expression of programmed death 1 (PD-1), cytotoxic T-lymphocyte antigen 4 (CTLA-4), and B and T lymphocyte attenuator (BTLA) (4). This altered expression results in poor T cell response and apoptosis, along with reduced interferon-gamma (IFN-gamma) production and increased pro-inflammatory cytokine release (4). Furthermore, the decrease in HLA-DR expression is also correlated with the decrease in CD14lowCD16+ inflammatory monocytes (5). Interestingly, COVID-19 patients also exhibit a reduction in HLA-DR that correlates with disease severity and immunosuppression (5).
References
1. Andersson G. (1998). Evolution of the human HLA-DR region. Frontiers in bioscience : a journal and virtual library. https://doi.org/10.2741/a317
2. Shiina, T., Hosomichi, K., Inoko, H., & Kulski, J. K. (2009). The HLA genomic loci map: expression, interaction, diversity and disease. Journal of human genetics. https://doi.org/10.1038/jhg.2008.5
3. Stern, L. J., & Calvo-Calle, J. M. (2009). HLA-DR: molecular insights and vaccine design. Current pharmaceutical design. https://doi.org/10.2174/138161209789105171
4. Zhuang, Y., Peng, H., Chen, Y., Zhou, S., & Chen, Y. (2017). Dynamic monitoring of monocyte HLA-DR expression for the diagnosis, prognosis, and prediction of sepsis. Frontiers in bioscience (Landmark edition). https://doi.org/10.2741/4547
5. Benlyamani, I., Venet, F., Coudereau, R., Gossez, M., & Monneret, G. (2020). Monocyte HLA-DR Measurement by Flow Cytometry in COVID-19 Patients: An Interim Review. Cytometry. Part A : the journal of the International Society for Analytical Cytology. https://doi.org/10.1002/cyto.a.24249
Long Name
Major Histocompatibility Complex Class II DR
Alternate Names
HLA-DRA, HLADR, MHC Class II DR
Gene Symbol
HLA-DRA
Additional HLA-DR Products
Product Documents for HLA-DR Antibody (L243) - Azide and BSA Free
Product Specific Notices for HLA-DR Antibody (L243) - Azide and BSA Free
This product is for research use only and is not approved for use in humans or in clinical diagnosis. Primary Antibodies are guaranteed for 1 year from date of receipt.
Loading...
Loading...
Loading...
Loading...
Loading...
![Immunohistochemistry: HLA-DR Antibody (L243) - Azide and BSA Free [NBP2-80773] Immunohistochemistry: HLA-DR Antibody (L243) - Azide and BSA Free [NBP2-80773]](https://resources.bio-techne.com/images/products/HLA-DR-Antibody-L243-Azide-and-BSA-Free-Immunohistochemistry-NBP2-80773-img0002.jpg)
![Flow Cytometry: HLA-DR Antibody (L243) - Azide and BSA Free [NBP2-80773] Flow Cytometry: HLA-DR Antibody (L243) - Azide and BSA Free [NBP2-80773]](https://resources.bio-techne.com/images/products/HLA-DR-Antibody-L243-Azide-and-BSA-Free-Flow-Cytometry-NBP2-80773-img0009.jpg)
![Flow (Cell Surface): HLA-DR Antibody (L243) - Azide and BSA Free [NBP2-80773] Flow (Cell Surface): HLA-DR Antibody (L243) - Azide and BSA Free [NBP2-80773]](https://resources.bio-techne.com/images/products/HLA-DR-Antibody-L243-Azide-and-BSA-Free-Flow-Cell-Surface-NBP2-80773-img0003.jpg)
![Flow Cytometry: HLA-DR Antibody (L243) - Azide and BSA Free [NBP2-80773] Flow Cytometry: HLA-DR Antibody (L243) - Azide and BSA Free [NBP2-80773]](https://resources.bio-techne.com/images/products/HLA-DR-Antibody-L243-Azide-and-BSA-Free-Flow-Cytometry-NBP2-80773-img0004.jpg)
![Flow Cytometry: HLA-DR Antibody (L243) - Azide and BSA Free [NBP2-80773] Flow Cytometry: HLA-DR Antibody (L243) - Azide and BSA Free [NBP2-80773]](https://resources.bio-techne.com/images/products/HLA-DR-Antibody-L243-Azide-and-BSA-Free-Flow-Cytometry-NBP2-80773-img0005.jpg)
![Flow Cytometry: HLA-DR Antibody (L243) - Azide and BSA Free [NBP2-80773] Flow Cytometry: HLA-DR Antibody (L243) - Azide and BSA Free [NBP2-80773]](https://resources.bio-techne.com/images/products/HLA-DR-Antibody-L243-Azide-and-BSA-Free-Flow-Cytometry-NBP2-80773-img0006.jpg)
![Flow Cytometry: HLA-DR Antibody (L243) - Azide and BSA Free [NBP2-80773] Flow Cytometry: HLA-DR Antibody (L243) - Azide and BSA Free [NBP2-80773]](https://resources.bio-techne.com/images/products/HLA-DR-Antibody-L243-Azide-and-BSA-Free-Flow-Cytometry-NBP2-80773-img0007.jpg)
![Flow Cytometry: HLA-DR Antibody (L243) - Azide and BSA Free [NBP2-80773] Flow Cytometry: HLA-DR Antibody (L243) - Azide and BSA Free [NBP2-80773]](https://resources.bio-techne.com/images/products/HLA-DR-Antibody-L243-Azide-and-BSA-Free-Flow-Cytometry-NBP2-80773-img0008.jpg)