Human ACE/CD143 PE-conjugated Antibody
R&D Systems, part of Bio-Techne | Catalog # FAB929P

Key Product Details
Species Reactivity
Validated:
Cited:
Applications
Validated:
Cited:
Label
Antibody Source
Product Specifications
Immunogen
aa 30-1261
Specificity
Clonality
Host
Isotype
Scientific Data Images for Human ACE/CD143 PE-conjugated Antibody
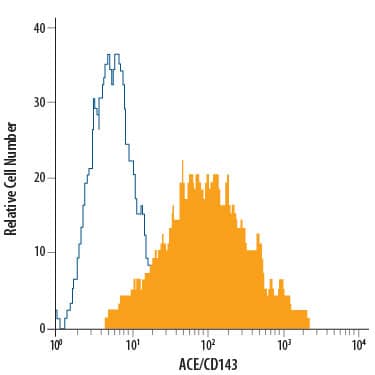
Detection of ACE/CD143 in Human monocyte-derived dendritic cells by Flow Cytometry.
Human monocyte-derived dendritic cells were stained with Mouse Anti-Human ACE/CD143 PE-conjugated Mono-clonal Antibody (Catalog # FAB929P, filled histogram) or isotype control antibody (Catalog # IC002P, open histogram). View our protocol for Staining Membrane-associated Proteins.Applications for Human ACE/CD143 PE-conjugated Antibody
Flow Cytometry
Sample: Human monocyte-derived dendritic cells
Formulation, Preparation, and Storage
Purification
Formulation
Shipping
Stability & Storage
- 12 months from date of receipt, 2 to 8 °C as supplied.
Background: ACE/CD143
ACE (also known as peptidyl-dipetidase A) is a zinc metallopeptidase important for blood pressure control and water and salt metabolism (2). It cleaves the C-terminal dipeptide from angiotensin I to produce the potent vasopressor octapeptide angiotensin II and inactivates bradykinin by the sequential removal of two C-terminal dipeptides. In addition to the two physiological substrates, ACE cleaves C-terminal dipeptides from various oligopeptides with a free C-terminus. Because of its location and specificity, ACE plays additional roles in immunity, reproduction and neuropeptide regulation. For example, ACE degrades Alzheimer amyloid beta-peptide (A beta), retards A beta aggregation, deposition, fibril formation, and inhibits cytotoxicity (3).
ACE is a type I membrane protein and exists in two isoforms (2). Somatic ACE, found in endothelial, epithelial and neuronal cells, comprises two highly similar domains called N- and C-domains, each of which contains the HExxH consensus sequence for zinc binding. Germinal ACE, found exclusively in the testes, comprises a single catalytically active domain identical to the C-domain of somatic ACE except for an N-terminal 67 residue germinal ACE-specific sequence. Physiological functions of the two tissue-specific isozymes are not interchangeable (4). For example, sperm-specific expression of the germinal ACE, not the somatic ACE, in ACE knockout male mice restored fertility.
Soluble ACE is present in many biological fluids, such as serum, seminal fluid, amniotic fluid and cerebrospinal fluid (2). The soluble ACE is derived from the membrane forms by actions of secretases or sheddases. The identities of the secretases have not been revealed, although they belong to the family of zinc metallopeptidases (5, 6).
References
- Soubrier, et al. (1988) Proc. Natl. Acad. Sci. USA 85:9386.
- Corvol, P. and T.A. Williams (1998) in Handbook of Proteolytic Enzymes. Barrett, A.J. et al. (eds): San Diego, Academic Press, p. 1066.
- Hu, et al. (2001) J. Biol. Chem. 276:47863.
- Kessler, et al. (2000) J. Biol. Chem. 275:26259.
- Eyries, et al. (2001) J. Biol. Chem. 276:5525.
- Alfalah, et al. (2001) J. Biol. Chem. 276:21105.
Long Name
Alternate Names
Gene Symbol
Additional ACE/CD143 Products
Product Documents for Human ACE/CD143 PE-conjugated Antibody
Product Specific Notices for Human ACE/CD143 PE-conjugated Antibody
For research use only