Human CD52 (Research Grade Alemtuzumab Biosimilar) Alexa Fluor® 405-conjugated Antibody
R&D Systems, part of Bio-Techne | Catalog # FAB9889V

Key Product Details
Species Reactivity
Applications
Label
Antibody Source
Product Specifications
Immunogen
Specificity
Clonality
Host
Isotype
Scientific Data Images
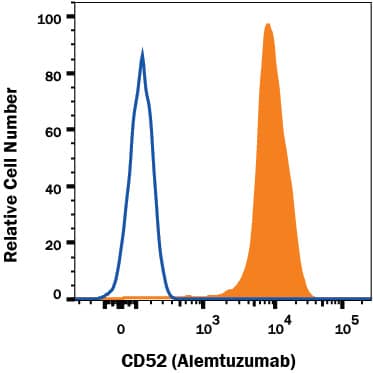
Detection of CD52 in human PBMC lymphocytes by Flow Cytometry.
Human PBMC lymphocytes were stained with Human Anti-Human CD52 (Alemtuzumab Biosimilar) Alexa Fluor® 405‑conjugated Monoclonal Antibody (Catalog # FAB9889V, filled histogram) or no primary antibody (open histogram). View our protocol for Staining Membrane-associated Proteins.Applications
Flow Cytometry
Sample: Human PBMC lymphocytes
Formulation, Preparation, and Storage
Purification
Formulation
Shipping
Stability & Storage
- 12 months from date of receipt, 2 to 8 °C as supplied.
Background: CD52
Alternate Names
Gene Symbol
Additional CD52 Products
Product Specific Notices
This product is provided under an agreement between Life Technologies Corporation and R&D Systems, Inc, and the manufacture, use, sale or import of this product is subject to one or more US patents and corresponding non-US equivalents, owned by Life Technologies Corporation and its affiliates. The purchase of this product conveys to the buyer the non-transferable right to use the purchased amount of the product and components of the product only in research conducted by the buyer (whether the buyer is an academic or for-profit entity). The sale of this product is expressly conditioned on the buyer not using the product or its components (1) in manufacturing; (2) to provide a service, information, or data to an unaffiliated third party for payment; (3) for therapeutic, diagnostic or prophylactic purposes; (4) to resell, sell, or otherwise transfer this product or its components to any third party, or for any other commercial purpose. Life Technologies Corporation will not assert a claim against the buyer of the infringement of the above patents based on the manufacture, use or sale of a commercial product developed in research by the buyer in which this product or its components was employed, provided that neither this product nor any of its components was used in the manufacture of such product. For information on purchasing a license to this product for purposes other than research, contact Life Technologies Corporation, Cell Analysis Business Unit, Business Development, 29851 Willow Creek Road, Eugene, OR 97402, Tel: (541) 465-8300. Fax: (541) 335-0354.
For research use only