Human MMR/CD206 APC-conjugated Antibody
R&D Systems, part of Bio-Techne | Catalog # FAB25342A

Key Product Details
Species Reactivity
Validated:
Cited:
Applications
Validated:
Cited:
Label
Antibody Source
Product Specifications
Immunogen
Leu19-Lys1383 (Thr399Ala) & (Leu407Phe)
Accession # P22897
Specificity
Clonality
Host
Isotype
Scientific Data Images for Human MMR/CD206 APC-conjugated Antibody
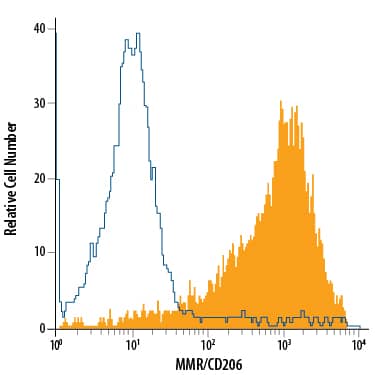
Detection of MMR/CD206 in Human Immature Dendritic Cells by Flow Cytometry.
Human monocyte-derived immature dendritic cells were stained with Mouse Anti-Human MMR/CD206 APC-conjugated Monoclonal Antibody (Catalog # FAB25342A, filled histogram) or isotype control antibody (Catalog # IC003A, open histogram). View our protocol for Staining Membrane-associated Proteins.Applications for Human MMR/CD206 APC-conjugated Antibody
Flow Cytometry
Sample: Human monocyte-derived immature dendritic cells
Formulation, Preparation, and Storage
Purification
Formulation
Shipping
Stability & Storage
- 12 months from date of receipt, 2 to 8 °C as supplied.
Background: MMR/CD206
The human Macrophage Mannose Receptor (MMR), also known as CD206 and MRC1 (mannose receptor C, type 1), is a 190 kDa scavenger receptor that is expressed on tissue macrophages, myeloid dendritic cells, and liver and lymphatic endothelial cells (1). It belongs to a family of receptors sharing similar protein structure that also includes DEC205, phospholipase A2 receptor, and Endo180 (2, 3). The human MMR protein is synthesized as a 1456 amino acid (aa) precursor that contains an 18 aa signal sequence, a 1371 aa extracellular region, a 21 aa transmembrane segment and a 46 aa cytoplasmic domain (4). Its extracellular region is composed of an N‑terminal cysteine-rich domain, followed by a single fibronectin type II repeat, and eight C-type lectin carbohydrate recognition domains (CRD) (3, 4). Human to mouse, the extracellular region is 82% aa identical. The cysteine-rich domain mediates recognition of sulfated N‑acetylgalactosamine, which occurs on some extracellular matrix proteins and is the terminal sugar of the unusual oligosaccharides present on pituitary hormones such as lutropin and thyrotropin (5). Several of the CRDs participate in the Ca2+-dependent recognition of carbohydrates showing a preference for branched sugars with terminal mannose, fucose or N‑acetylglucosamine (6). The cytoplasmic domain of MMR includes a tyrosine-based motif for internalization in clathrin‑coated vesicles. Once internalized, ligands are released following acidification of phagosomes or endosomes, and the receptor is recycled to the cell surface (3, 7). MMR mediates phagocytosis upon binding to target structures that occur on a variety of pathogenic microorganisms including Gram-negative and Gram-positive bacteria, yeasts, parasites, and mycobacteria. MMR also functions to maintain homeostasis through the endocytosis of potentially harmful glycoproteins associated with inflammation (2, 3).
References
- East, L. and C. Isake (2002) Biochim. Biophys. Acta 1572:364.
- Chieppa, M. et al. (2003) J. Immunol. 171:4552.
- Figdor, C. et al. (2002) Nat. Rev. Immunol. 2:77.
- Taylor, M. et al. (1990) J. Biol. Chem. 265:12156.
- Leteux, C. et al. (2000) J. Exp. Med. 191:1117.
- Martinez-Pomares, L. et al. (2001) Immunobiology 204:527.
- Feinberg, H. et al. (2000) J. Biol. Chem. 275:21539.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional MMR/CD206 Products
Product Documents for Human MMR/CD206 APC-conjugated Antibody
Product Specific Notices for Human MMR/CD206 APC-conjugated Antibody
For research use only