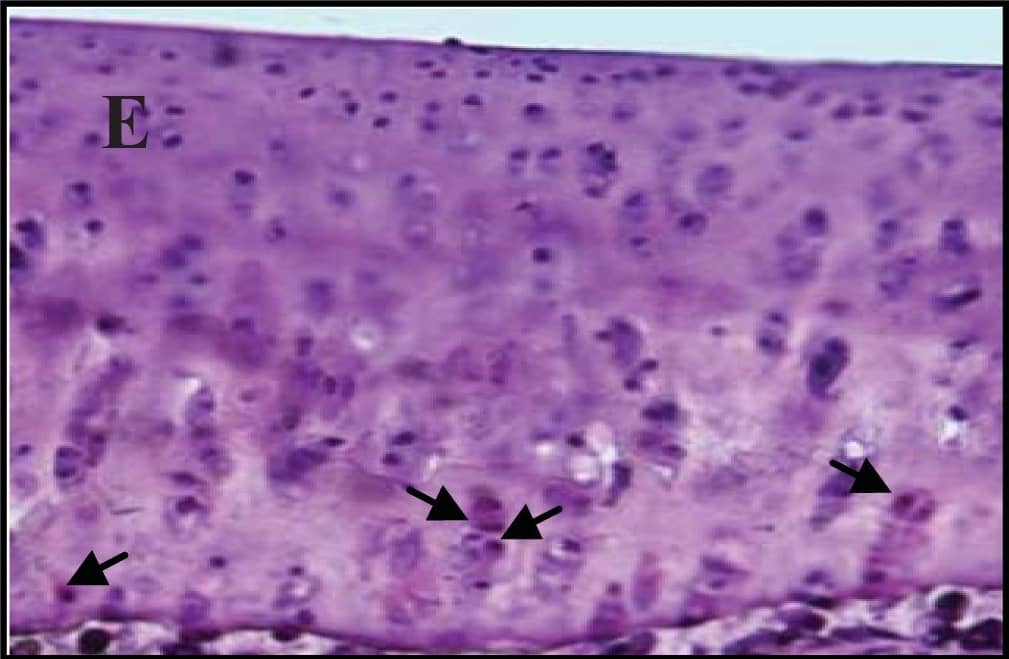
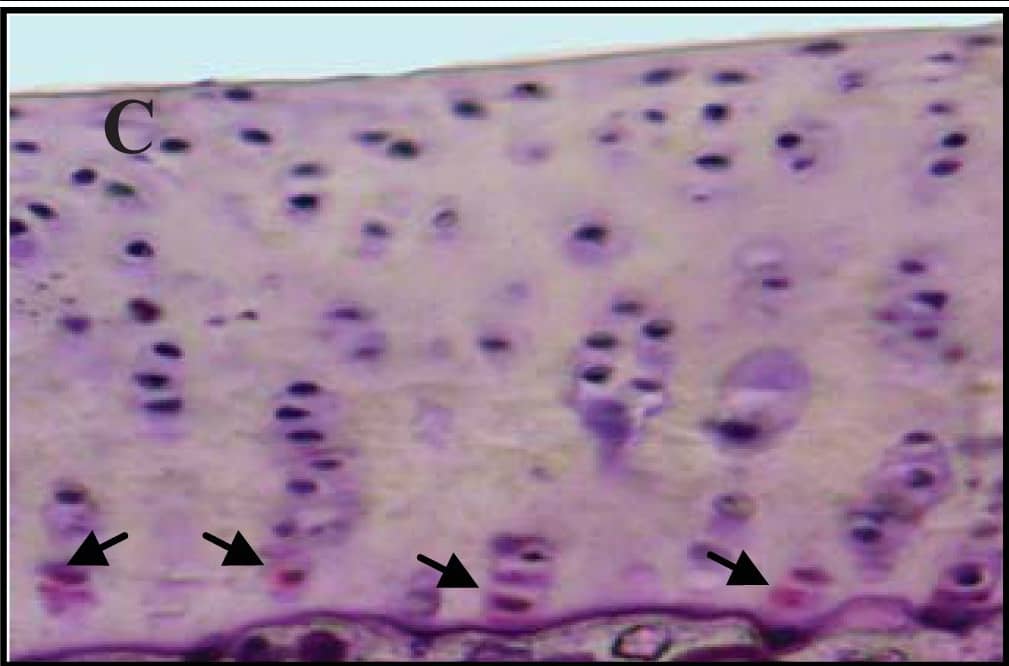
Detection of Guinea Pig Caspase-3 by Immunohistochemistry
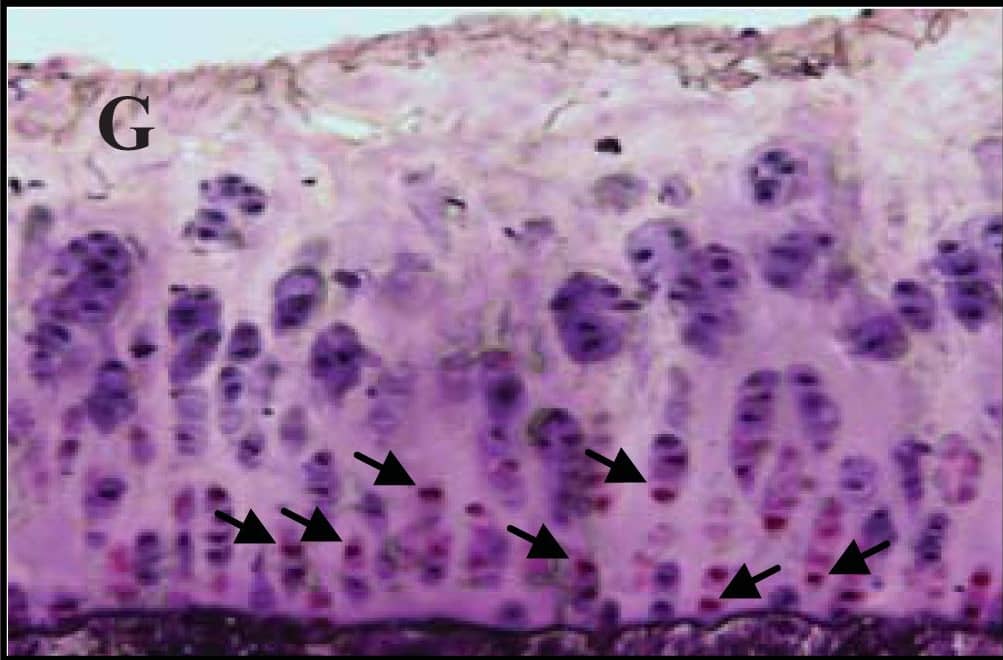
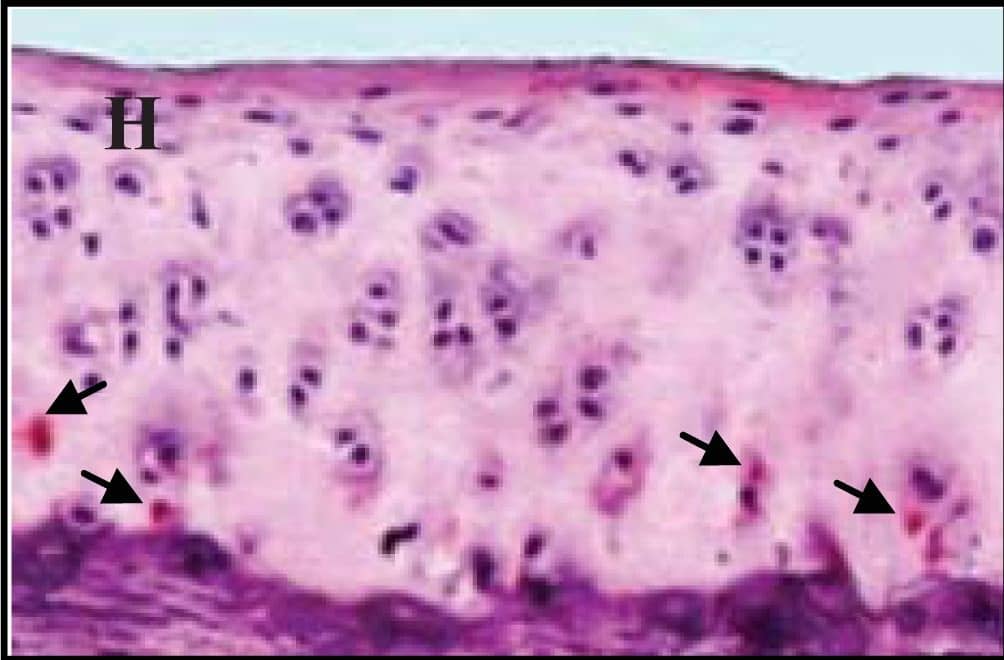
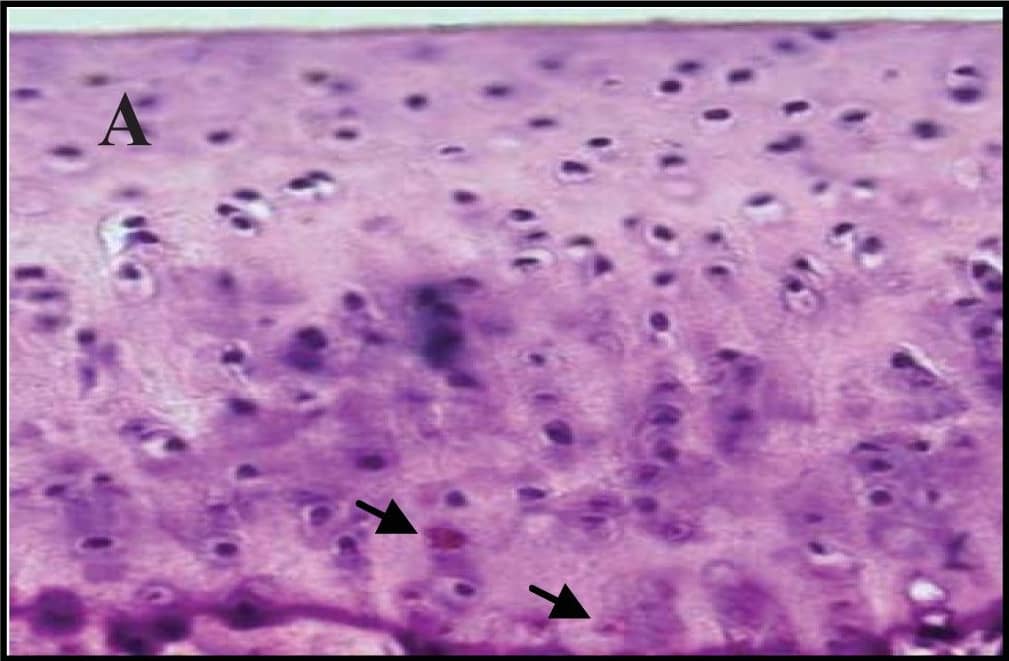
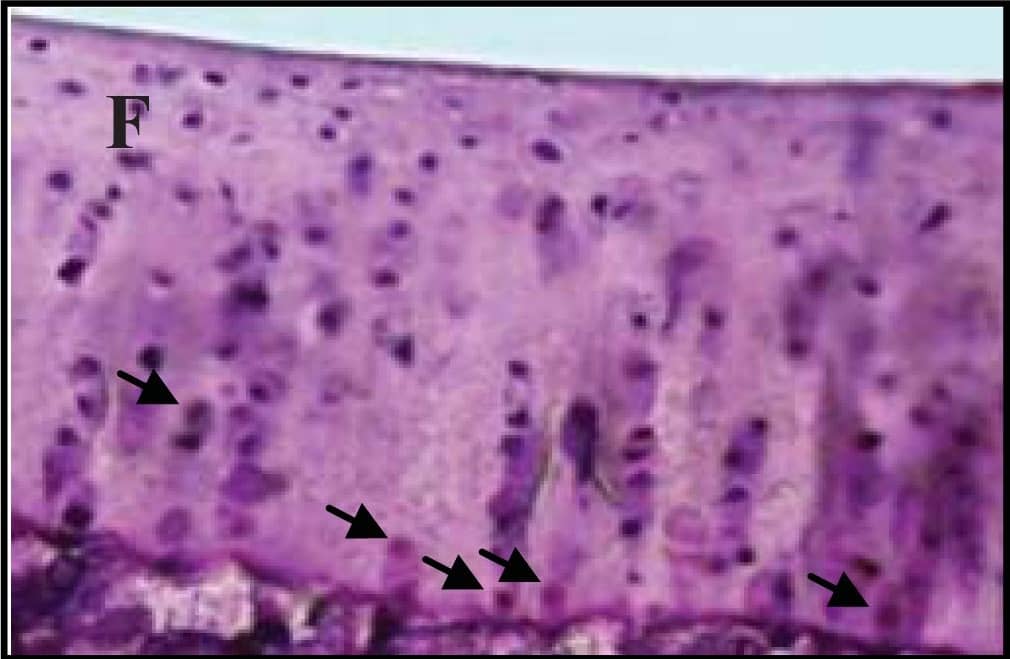
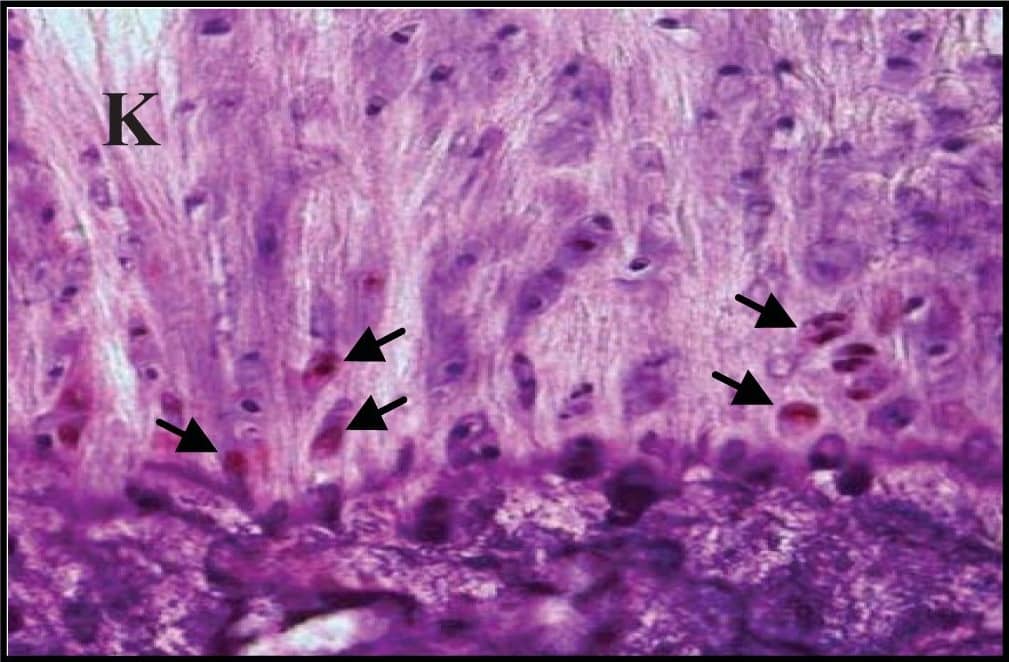
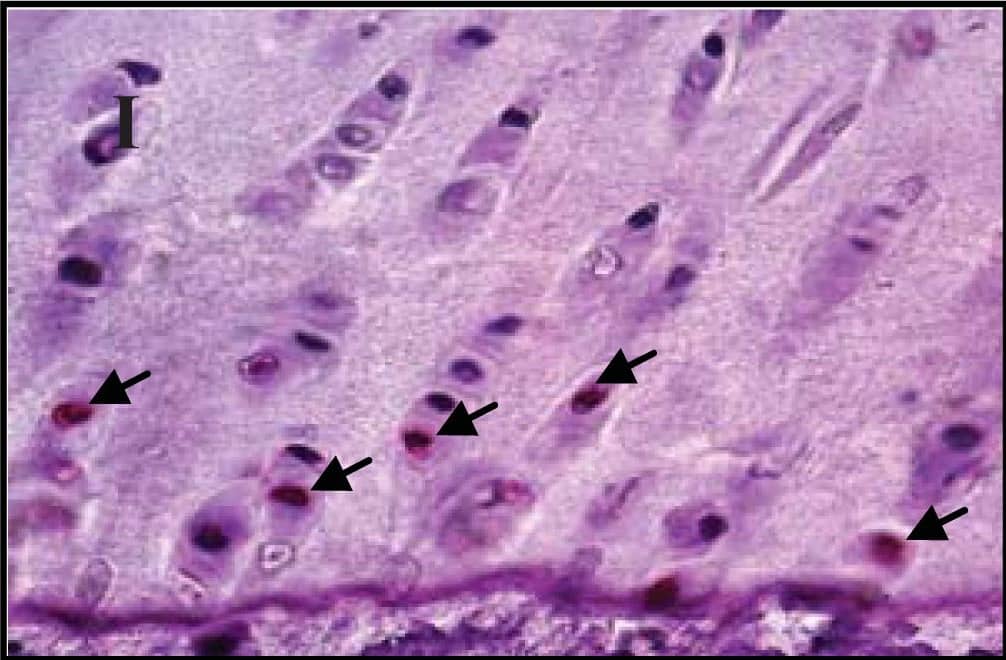
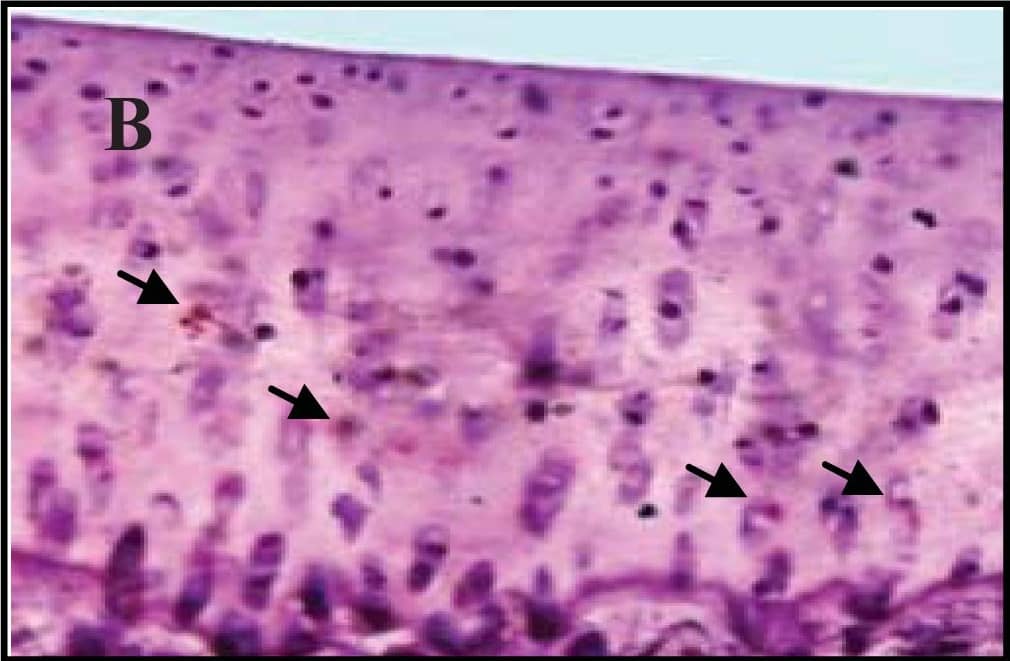
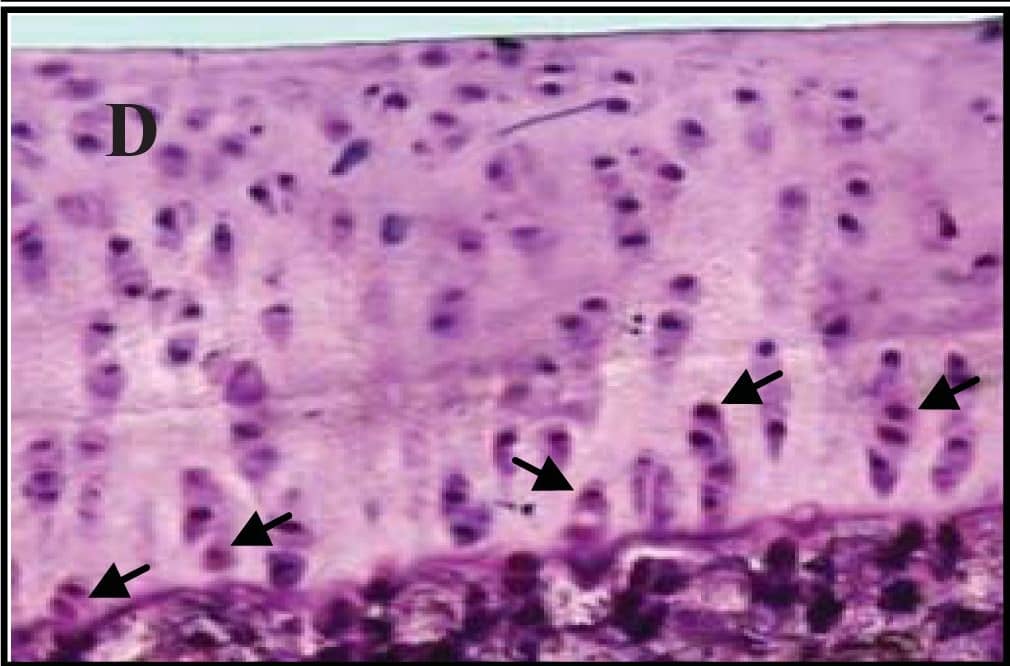
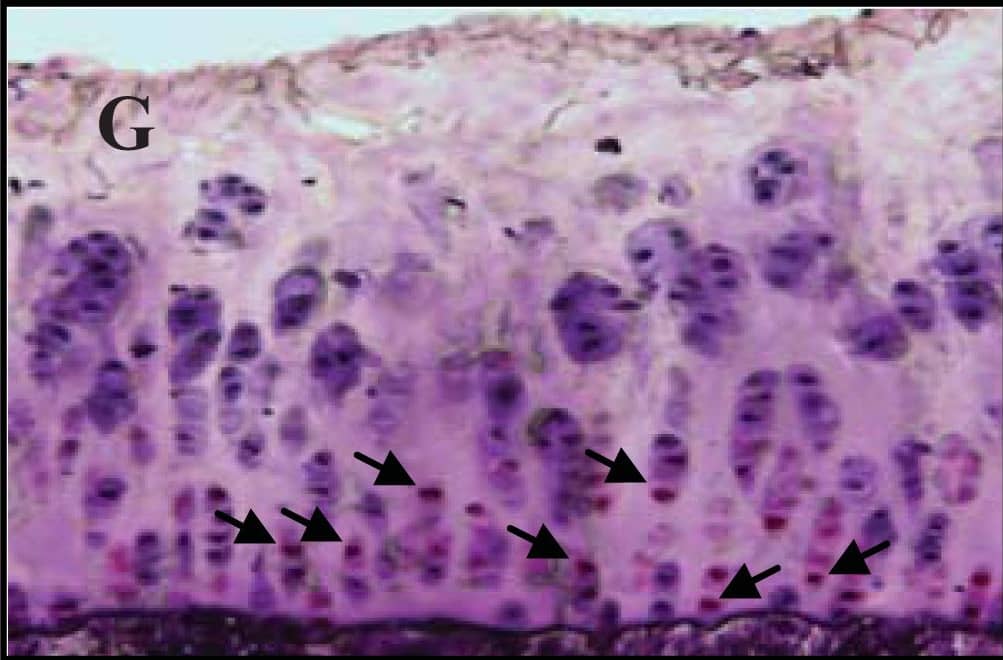
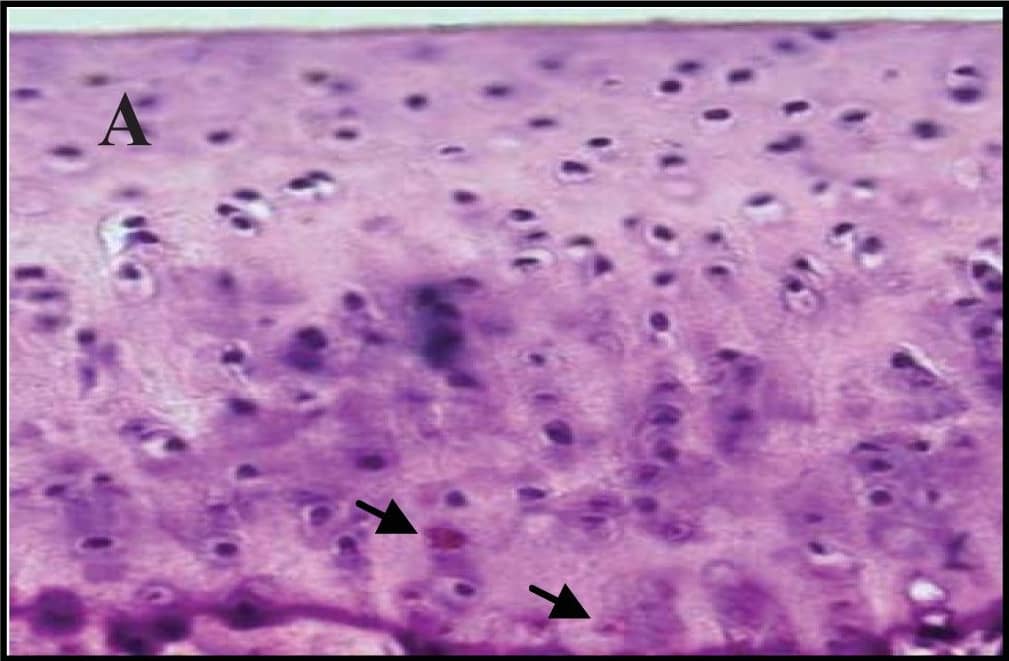
Representative sections of the medial side of right tibial epiphysis stained with/without caspase-3. Sections stained with caspase-3 antibody at four different time points (DH: A, C, E and G; BS2: B, D, F and H); a higher magnification (I); the growth plate (J) and fibrocartilage of anterior cruciate ligament (K) show caspase-3 positive cells (red cytoplasmic staining; arrow). A negative control section is shown in (L). Image collected and cropped by CiteAb from the following publication (https://www.mdpi.com/1422-0067/14/9/17729), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Guinea Pig Caspase-3 by Immunohistochemistry
Representative sections of the medial side of right tibial epiphysis stained with/without caspase-3. Sections stained with caspase-3 antibody at four different time points (DH: A, C, E and G; BS2: B, D, F and H); a higher magnification (I); the growth plate (J) and fibrocartilage of anterior cruciate ligament (K) show caspase-3 positive cells (red cytoplasmic staining; arrow). A negative control section is shown in (L). Image collected and cropped by CiteAb from the following publication (https://www.mdpi.com/1422-0067/14/9/17729), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Caspase-3 by Immunohistochemistry
Immunohistochemical staining for caspase 3 on surgically transected tumours. A) Epo-treated tumour 24 hours after surgery. B) Placebo-treated tumour 24 hours after surgery. C) Placebo-treated tumour 48 hours after surgery Image collected and cropped by CiteAb from the following publication (https://bmccancer.biomedcentral.com/articles/10.1186/1471-2407-14-648), licensed under a CC-BY license. Not internally tested by R&D Systems.
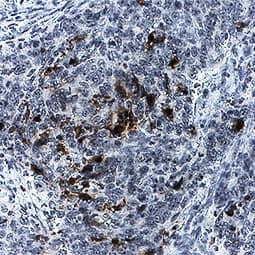
Detection of Mouse Caspase-3 by Immunohistochemistry
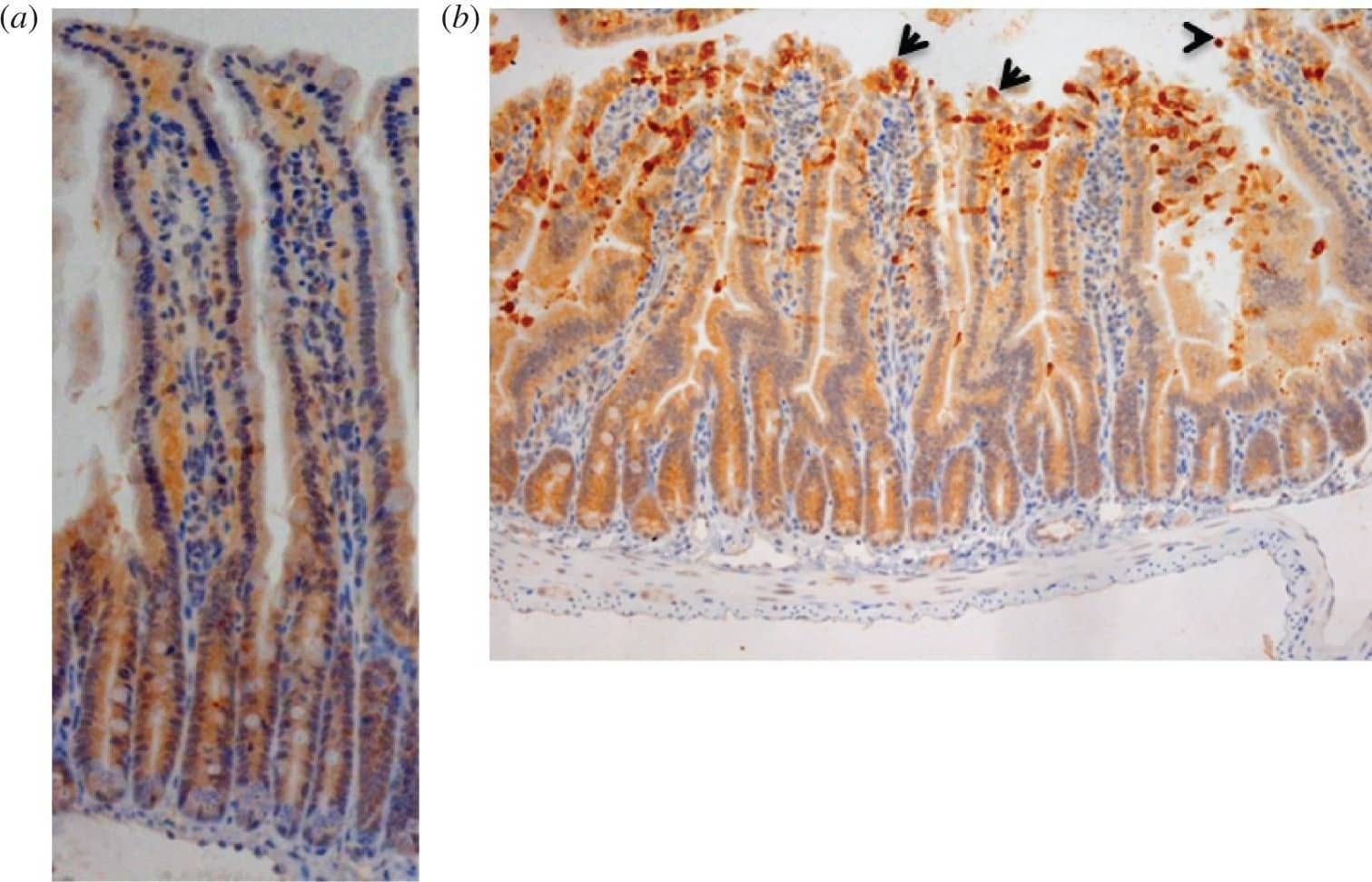
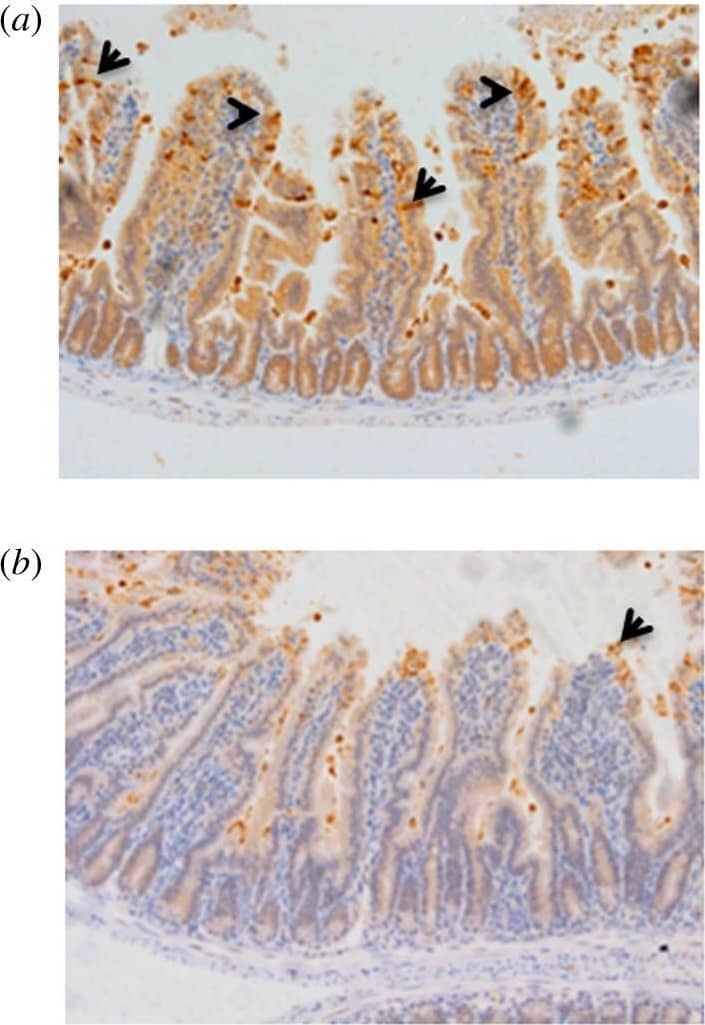
LPS challenge induces cell shedding from small intestinal villi. C57 BL/6 mice were administered either (a) PBS (control) or (b) LPS by IP injection and proximal small intestines removed after 1.5 h. Processed tissue was sectioned and stained by immunohistochemistry for CC3 (i.e. brown cells indicate shedding event), also highlighted by arrows. A representative picture for each group is shown (12 mice per group, two independent experiments). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28123052), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Guinea Pig Caspase-3 by Immunohistochemistry
Representative sections of the medial side of right tibial epiphysis stained with/without caspase-3. Sections stained with caspase-3 antibody at four different time points (DH: A, C, E and G; BS2: B, D, F and H); a higher magnification (I); the growth plate (J) and fibrocartilage of anterior cruciate ligament (K) show caspase-3 positive cells (red cytoplasmic staining; arrow). A negative control section is shown in (L). Image collected and cropped by CiteAb from the following publication (https://www.mdpi.com/1422-0067/14/9/17729), licensed under a CC-BY license. Not internally tested by R&D Systems.
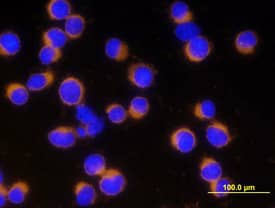
Detection of Mouse Caspase-3 by Immunocytochemistry/Immunofluorescence
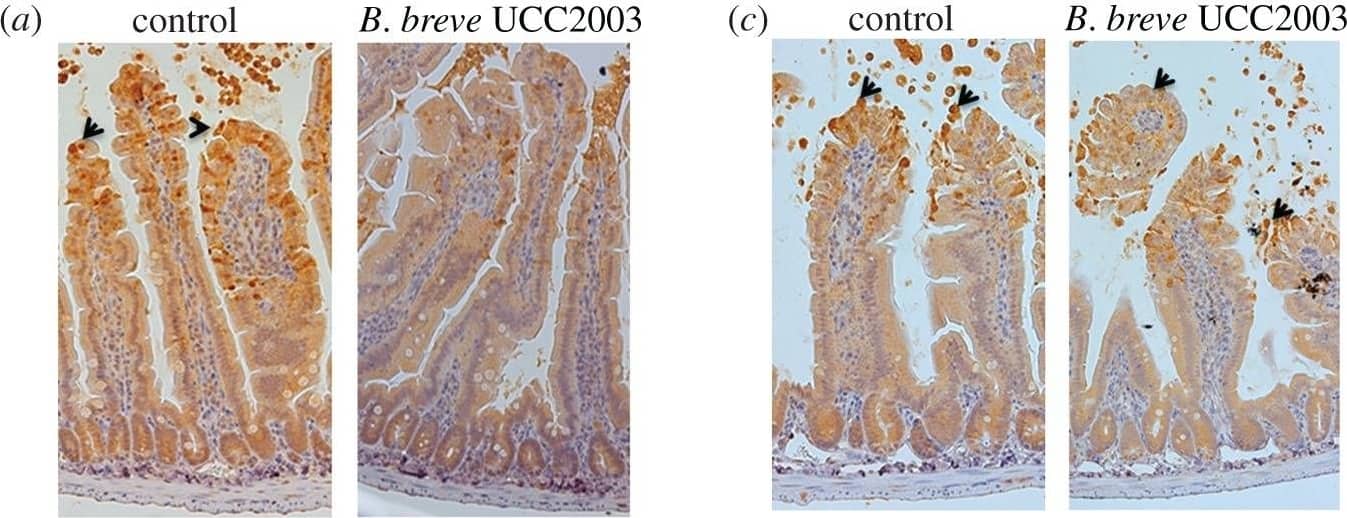
The cytoprotective effect of B. breve is MyD88 dependent. (a,b) IEC MyD88+/+ mice and (c,d) IEC MyD88−/− mice were gavaged with PBS (control) or B. breve UCC2003 and challenged with LPS. Paraffin-embedded intestinal sections were stained with anti-CC3 and quantified using the WinCrypts and Score programs. (e) Columns shown TLR2 expression via RT-PCR. Data are mean ± s.d., n = 12 (two independent experiments) analysed with Mann–Whitney U-test. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28123052), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Guinea Pig Caspase-3 by Immunohistochemistry
Representative sections of the medial side of right tibial epiphysis stained with/without caspase-3. Sections stained with caspase-3 antibody at four different time points (DH: A, C, E and G; BS2: B, D, F and H); a higher magnification (I); the growth plate (J) and fibrocartilage of anterior cruciate ligament (K) show caspase-3 positive cells (red cytoplasmic staining; arrow). A negative control section is shown in (L). Image collected and cropped by CiteAb from the following publication (https://www.mdpi.com/1422-0067/14/9/17729), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Guinea Pig Caspase-3 by Immunohistochemistry
Representative sections of the medial side of right tibial epiphysis stained with/without caspase-3. Sections stained with caspase-3 antibody at four different time points (DH: A, C, E and G; BS2: B, D, F and H); a higher magnification (I); the growth plate (J) and fibrocartilage of anterior cruciate ligament (K) show caspase-3 positive cells (red cytoplasmic staining; arrow). A negative control section is shown in (L). Image collected and cropped by CiteAb from the following publication (https://www.mdpi.com/1422-0067/14/9/17729), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Guinea Pig Caspase-3 by Immunohistochemistry
Representative sections of the medial side of right tibial epiphysis stained with/without caspase-3. Sections stained with caspase-3 antibody at four different time points (DH: A, C, E and G; BS2: B, D, F and H); a higher magnification (I); the growth plate (J) and fibrocartilage of anterior cruciate ligament (K) show caspase-3 positive cells (red cytoplasmic staining; arrow). A negative control section is shown in (L). Image collected and cropped by CiteAb from the following publication (https://www.mdpi.com/1422-0067/14/9/17729), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Caspase-3 by Immunocytochemistry/Immunofluorescence
Bifidobacterium breve UCC2003 protects against LPS-induced cell shedding. C57 BL/6 mice received three daily oral gavage doses of (a) PBS or (b) approximately 1 × 109B. breve UCC2003 followed by IP challenge with LPS 24 h later. Representative images are shown. Formalin-fixed, paraffin-embedded intestinal sections were sectioned and stained with anti-CC3 and (c) quantified using the WinCrypts and Score programs, 20 well-orientated hemi-villi were counted per mouse. Data are mean ± s.d., n = 12 (two independent experiments) analysed with a Mann–Whitney U-test. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28123052), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Guinea Pig Caspase-3 by Immunohistochemistry
Representative sections of the medial side of right tibial epiphysis stained with/without caspase-3. Sections stained with caspase-3 antibody at four different time points (DH: A, C, E and G; BS2: B, D, F and H); a higher magnification (I); the growth plate (J) and fibrocartilage of anterior cruciate ligament (K) show caspase-3 positive cells (red cytoplasmic staining; arrow). A negative control section is shown in (L). Image collected and cropped by CiteAb from the following publication (https://www.mdpi.com/1422-0067/14/9/17729), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Caspase-3 by Immunocytochemistry/Immunofluorescence
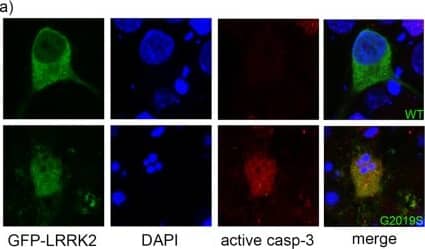
Genetic deletion of Bax prevents mutant LRRK2 induced neuronal death. Primary embryonic cortical neurons are derived from WT mice or mice lacking the pro-apoptotic protein Bax, and transiently transfected with GFP-tagged WT or mutant LRRK2 (R1441C, G2019S). (a) A representative image showing typical morphological features of apoptotic neurons expressing mutant LRRK2; that are positive for active caspase-3 (casp3) and display condensed and fragmented nuclei. (b) Quantification of apoptotic neuronal death (apoptotic nuclear profiles, “apo”; and active caspase-3 positive neurons, “casp3”) from cultures depicted in (a); **p < 0.01 in comparison to WT transfected neurons. (c) Primary neurons were prepared from WT mice or mice deficient in Bax, and treated with the topoisomerase I inhibitor camptothecin (“cam”, 5 μM, 18 hr), or doxorubicin (“doxo”, 10 ng/ml, 36 hr). Cells were fixed and processed for apoptotic nuclear counts. (d) Primary cortical neurons were prepared from WT mice or mice deficient in Bax, and transiently transfected with WT, R1441C, or G2019S LRRK2. As before, the cultures were fixed and the percentage of LRRK2-positive neurons that displayed apoptotic nuclear features was determined. The absence of Bax significantly protected neurons from death induced by mutant LRRK2. ***p < 0.001 compared to WT neurons expressing R1441C- or G2019S-LRRK2. Each data point represents the mean +/− SEM from 3–4 independent transfections from a representative culture. Cultures were repeated at least 3 times with similar differences. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/29472595), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Caspase-3 by Immunocytochemistry/Immunofluorescence
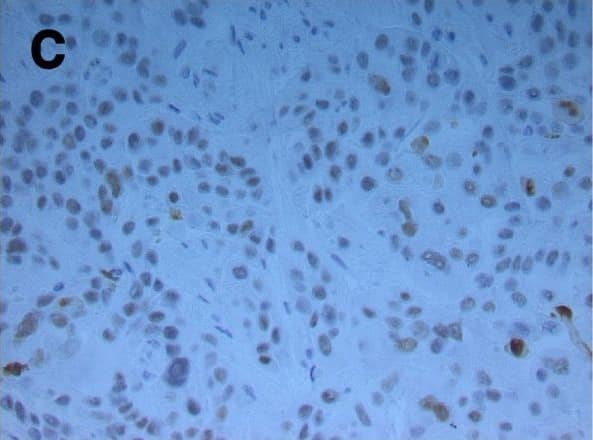
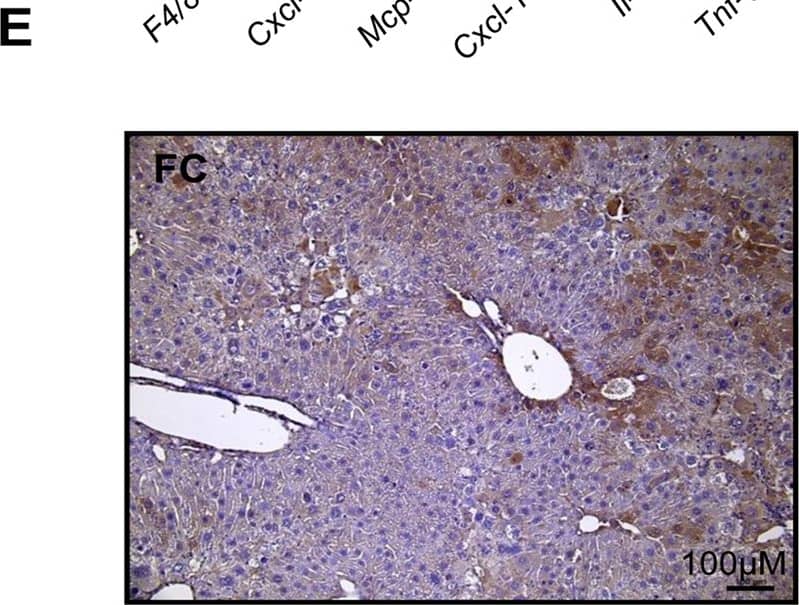
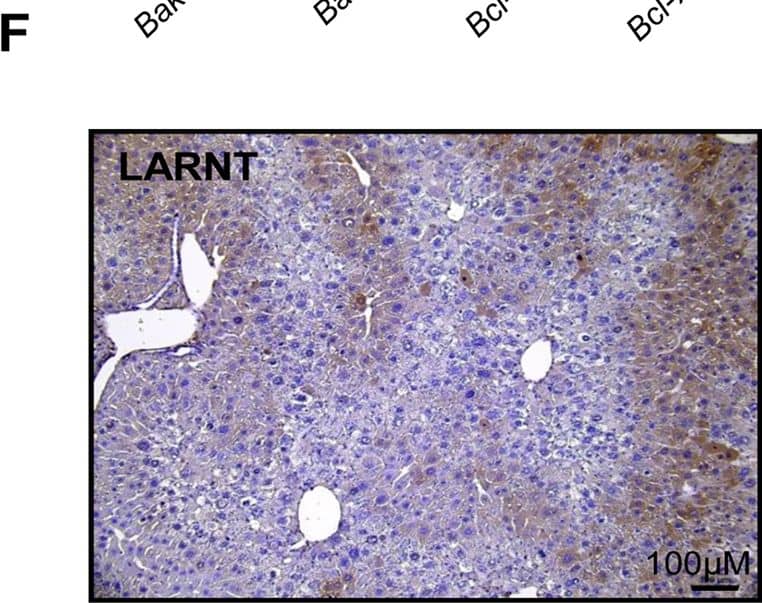
Hepatocyte ARNT deletion alters fibrotic and apoptotic gene expression in the TAA model of fibrosis.Results for FC are shown in black columns and LARNT in white columns. Results are expressed relative to FC level. (A) Messenger RNA expression of ARNT and collagen isotypes. (B) Expression of genes regulating fibrosis in FC and LARNT mice. (C) F4/80 and inflammatory cytokine expression in FC and LARNT mice. (D) Apoptotic gene expression in FC and LARNT mice. (E) Representative histology of FC liver stained with anti-Caspase 3 at 100X magnification. (F) Representative histology of LARNT liver stained with anti-Caspase 3 at 100X magnification. (G) Average Caspase 3 positive cell counts per field of view at 100 X magnification. Mean±SEM, * = p<0.05 ** = p<0.01, *** = p<0.001. N = 5-6/group. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0121650), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Caspase-3 by Immunocytochemistry/Immunofluorescence
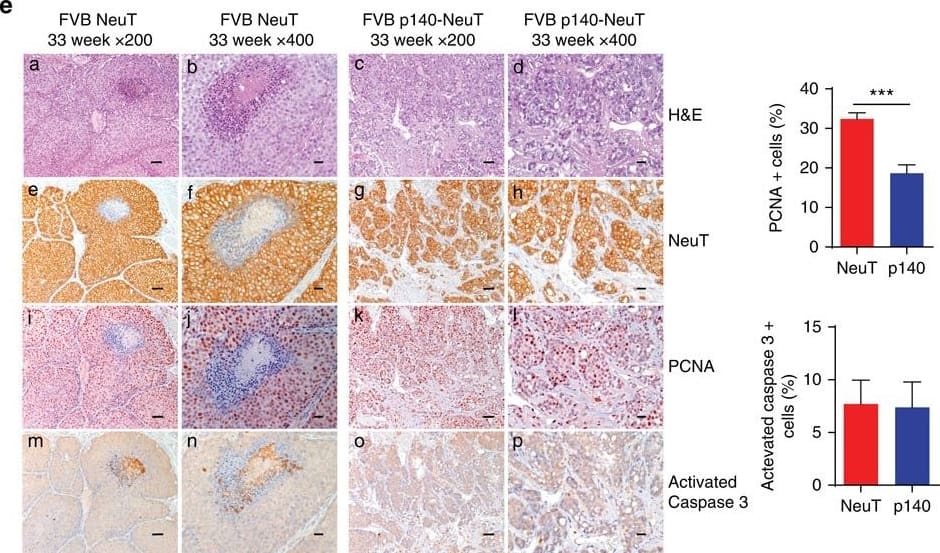
p140Cap expression is causative in limiting tumour growth in NeuT mice.(a) Expression cassette used for the generation of MMTV-p140Cap transgenic mice. The Myc epitope is inserted at the carboxyterminal region of the protein. (b) Extracts of tumours derived from NeuT mice (a,b) or p140-NeuT mice (c,d) were run on 6% SDS–PAGE and stained with antibodies to p140Cap, ERBB2 and tubulin for loading control. (c) Percentage of tumour free mice in NeuT (red line) and p140-NeuT (blue-dashed line) transgenic animals in both FVB (left) or BALB/c background (right). Twelve mice were analysed for each group. Fisher's exact test, Two sided, P=0,0022; P=0,0056. Error bar: s.e.m. (d) Total tumour burden in NeuT (red) and p140-NeuT (blue) mice in both the FVB (left) or BALB/c backgrounds (right) was measured. Ten mice were analysed in each group. Unpaired t test: (*P<0.05; ***P<0.001). Error bar: s.e.m. (e) Paraffin-embedded sections from three NeuT and three p140-NeuT tumours taken from mice at 33 weeks of age were analysed for hematoxylin–eosin (H&E) (a–d) and for immunohistochemistry with antibodies to NeuT (e–h), PCNA (i–l) and activated Caspase3 (m–p). Representative images are shown. Scale bar, 50 μm (first and third columns); 20μm (second and fourth columns). Histograms on the right show the percentage of PCNA+ (upper panel) and Activated Caspase3+ (lower panel) cells. Statistical significative differences were evaluated using unpaired t-tests (***P<0.001). Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/ncomms14797), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Caspase-3 by Immunocytochemistry/Immunofluorescence
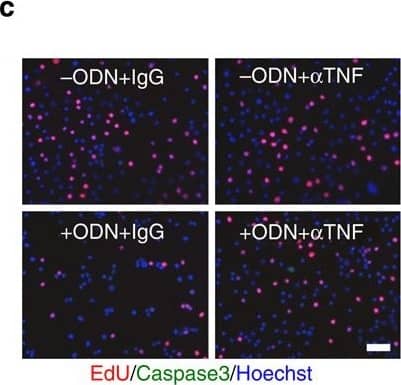
Microglia-derived TNF-alpha alleviates aberrant neurogenesis.(a) qRT-PCR analyses of Tnf-alpha levels in the DG of WT and TLR9 KO mice at the indicated time points after seizure. Experimental controls were KA-untreated WT and TLR9 KO mice, respectively (n=3 animals). *P<0.05 by analysis of variance (ANOVA) with Tukey post-hoc tests. (b) Experimental scheme for assessing aNS/PC proliferation in microglia culture-derived CM pretreated with TNF-alpha -neutralizing antibody or IgG control. (c) Representative images of EdU (red), active caspase3 (green) and Hoechst (blue) staining in aNS/PCs cultured with the indicated CM from microglia (n=4 experiments). Scale bar, 50 μm. (d) Quantification of EdU+ or active caspase3+ cells in c (n=4 experiments). (e) Experimental timeline for assessing aNS/PC proliferation in thalidomide (Thal)-treated mice. (f) Representative images of BrdU+DCX+ newly generated immature neurons in the DG (n=4 animals). Scale bar, 50 μm. (g,h) Quantification of the number of BrdU+DCX+ cells in f, in the DG (g) and in the hilus (h) (n=4 animals). *P<0.05 and **P<0.01 by ANOVA with Tukey post-hoc tests. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/25751136), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human Caspase-3 by Immunocytochemistry/Immunofluorescence
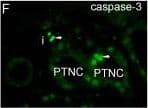
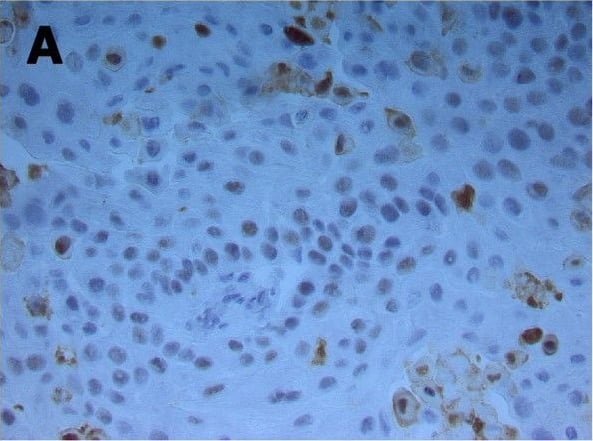
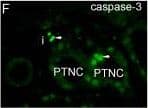
Double immunofluorescent staining of FSGS kidneys to Ki-67, alpha-tubulin and DAPI or double immunofluorescent staining to caspase-3 and DAPI. A. FSGS kidneys: cysts with simple squamous epithelium (CS) filled with colloid content, interstitium (i) and collecting tubules (ct) contain Ki-67-positive cells (arrows)(square). B. alpha-tubulin visualizes short cilia (arrows) in the cysts and extremely long cilia in collecting tubules (arrows)(square). C. Nuclear DAPI stain. D. Merging of A + B + C shows relationships between proliferating cells (green) and cilia (red). Immunostaining to Ki-67, alpha-tubulin and DAPI; scale bar 10 μm. E. Distribution of Ki-67 positive cells (%) in CNF and FSGS nephrotic syndrome. Cysts of proximal tubules with simple squamous epithelium (CS) or simple cuboidal epithelium (CC), proximal tubules with apparently normal simple cuboidal/columnar epithelium (PTNC). Data are shown as mean ± SD. Significant differences (Kruskal–Wallis) indicated by ***p < 0.0001. F. FSGS kidneys: caspase-3-positive apoptotic cells (arrows) in proximal tubules (PTNC) and interstitium (i). G. Nuclear DAPI stain. H. Merging of F + G shows green caspase-3- positive apoptotic nuclei and blue non-apoptotic nuclei. Immunostaining to caspase-3 and DAPI, scale bar 10 μm. Image collected and cropped by CiteAb from the following publication (https://bmcnephrol.biomedcentral.com/articles/10.1186/1471-2369-15-3), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Caspase-3 by Immunohistochemistry
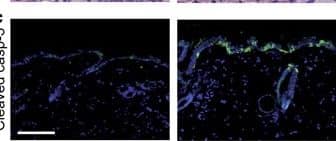
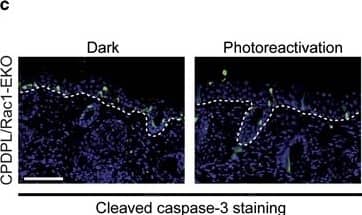
Epidermis-specific deletion of Rac1 increases UV-light-induced keratinocyte apoptosis in vivo. (a) H/E staining of UV-irradiated skin of Rac1 fl/fl and Rac1-EKO mice at 12 h after UV-irradiation. Black arrows indicate sunburn cells. (b) Graph shows the percentage number of sunburn cells at 12 h with (red bars) or without (blue bars) UV-irradiation in Rac1 fl/fl (n=4) and Rac1-EKO (n=5) mice. The percentage of sunburn cells within the epidermis after UV-irradiation in Rac1 fl/fl mice was 3.4%, whereas in Rac1-EKO mice it was 8.6% of total epidermal keratinocytes. Non-irradiated samples showed <1% sunburn cells in both the genotypes. (c) Immunostainings against cleaved caspase-3 (green) of UV-irradiated skin of Rac1 fl/fl and Rac1-EKO mice at 12 h after UV-irradiation. Nuclei are stained in blue. Scale bar=100 μm. (d) Western blot analysis of cleaved caspase-3 from epidermal lysates of untreated (no UV) and UV-light treated (UV) Rac1 fl/fl and Rac1-EKO mice. Non-irradiated controls showed no bands for cleaved capsase-3, whereas samples of irradiated epidermis showed cleaved caspase-3-specific bands at ~25 kDa and 23 kDa. Numbers on the left denote molecular weights in kDa. (e) Graph shows densitometry analysis of western blot in (d). Error bars show S.D. Asterisks show P-value<0.001 Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28277539), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Caspase-3 by Immunocytochemistry/Immunofluorescence
Hepatocyte ARNT deletion alters fibrotic and apoptotic gene expression in the TAA model of fibrosis.Results for FC are shown in black columns and LARNT in white columns. Results are expressed relative to FC level. (A) Messenger RNA expression of ARNT and collagen isotypes. (B) Expression of genes regulating fibrosis in FC and LARNT mice. (C) F4/80 and inflammatory cytokine expression in FC and LARNT mice. (D) Apoptotic gene expression in FC and LARNT mice. (E) Representative histology of FC liver stained with anti-Caspase 3 at 100X magnification. (F) Representative histology of LARNT liver stained with anti-Caspase 3 at 100X magnification. (G) Average Caspase 3 positive cell counts per field of view at 100 X magnification. Mean±SEM, * = p<0.05 ** = p<0.01, *** = p<0.001. N = 5-6/group. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0121650), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Caspase-3 by Immunohistochemistry
Immunohistochemical staining for caspase 3 on surgically transected tumours. A) Epo-treated tumour 24 hours after surgery. B) Placebo-treated tumour 24 hours after surgery. C) Placebo-treated tumour 48 hours after surgery Image collected and cropped by CiteAb from the following publication (https://bmccancer.biomedcentral.com/articles/10.1186/1471-2407-14-648), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Guinea Pig Caspase-3 by Immunohistochemistry
Representative sections of the medial side of right tibial epiphysis stained with/without caspase-3. Sections stained with caspase-3 antibody at four different time points (DH: A, C, E and G; BS2: B, D, F and H); a higher magnification (I); the growth plate (J) and fibrocartilage of anterior cruciate ligament (K) show caspase-3 positive cells (red cytoplasmic staining; arrow). A negative control section is shown in (L). Image collected and cropped by CiteAb from the following publication (https://www.mdpi.com/1422-0067/14/9/17729), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Caspase-3 by Western Blot
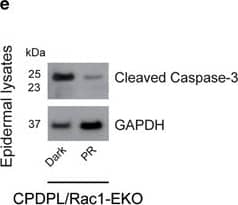
DNA damage has an important role in increased UV-light-induced keratinocyte apoptosis in Rac1-EKO epidermis in vivo. (a and c) H/E stainings and immunostainings against cleaved caspase-3 (green) of UV-irradiated skin of CPDPL/Rac1-EKO mice kept in the dark and under the photoreactivation lamp. Nuclei are stained in blue. Dashed line in c demarcates border between epidermis and dermis. Scale bar=100 μm. (e) Western blot analysis of cleaved caspase-3 from epidermal lysates of UV-irradiated CPDPL/Rac1-EKO mice kept in the dark and under the photoreactivation lamp. GAPDH was used as a loading control. Numbers on the left denote molecular weight in kDa. (b,d, and f) Graphs show quantification of sunburn cells (n=5, 4) (b), cleaved caspase-3 positive cells (n=9, 8) (d) and densitometric analysis of cleaved caspase-3 western blots (n=4, 6) (f) from UV-irradiated CPDPL/Rac1-EKO mice kept in the dark (black bars) and under the photoreactivation (PR) lamp (gray bars). Error bars show S.D. Asterisks show P-value. **<0.01, ***<0.001 Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28277539), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Guinea Pig Caspase-3 by Immunohistochemistry
Representative sections of the medial side of right tibial epiphysis stained with/without caspase-3. Sections stained with caspase-3 antibody at four different time points (DH: A, C, E and G; BS2: B, D, F and H); a higher magnification (I); the growth plate (J) and fibrocartilage of anterior cruciate ligament (K) show caspase-3 positive cells (red cytoplasmic staining; arrow). A negative control section is shown in (L). Image collected and cropped by CiteAb from the following publication (https://www.mdpi.com/1422-0067/14/9/17729), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Caspase-3 by Immunohistochemistry
DNA damage has an important role in increased UV-light-induced keratinocyte apoptosis in Rac1-EKO epidermis in vivo. (a and c) H/E stainings and immunostainings against cleaved caspase-3 (green) of UV-irradiated skin of CPDPL/Rac1-EKO mice kept in the dark and under the photoreactivation lamp. Nuclei are stained in blue. Dashed line in c demarcates border between epidermis and dermis. Scale bar=100 μm. (e) Western blot analysis of cleaved caspase-3 from epidermal lysates of UV-irradiated CPDPL/Rac1-EKO mice kept in the dark and under the photoreactivation lamp. GAPDH was used as a loading control. Numbers on the left denote molecular weight in kDa. (b,d, and f) Graphs show quantification of sunburn cells (n=5, 4) (b), cleaved caspase-3 positive cells (n=9, 8) (d) and densitometric analysis of cleaved caspase-3 western blots (n=4, 6) (f) from UV-irradiated CPDPL/Rac1-EKO mice kept in the dark (black bars) and under the photoreactivation (PR) lamp (gray bars). Error bars show S.D. Asterisks show P-value. **<0.01, ***<0.001 Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28277539), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Guinea Pig Caspase-3 by Immunohistochemistry
Representative sections of the medial side of right tibial epiphysis stained with/without caspase-3. Sections stained with caspase-3 antibody at four different time points (DH: A, C, E and G; BS2: B, D, F and H); a higher magnification (I); the growth plate (J) and fibrocartilage of anterior cruciate ligament (K) show caspase-3 positive cells (red cytoplasmic staining; arrow). A negative control section is shown in (L). Image collected and cropped by CiteAb from the following publication (https://www.mdpi.com/1422-0067/14/9/17729), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Caspase-3 by Immunohistochemistry
Immunohistochemical staining for caspase 3 on surgically transected tumours. A) Epo-treated tumour 24 hours after surgery. B) Placebo-treated tumour 24 hours after surgery. C) Placebo-treated tumour 48 hours after surgery Image collected and cropped by CiteAb from the following publication (https://bmccancer.biomedcentral.com/articles/10.1186/1471-2407-14-648), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Guinea Pig Caspase-3 by Immunohistochemistry
Representative sections of the medial side of right tibial epiphysis stained with/without caspase-3. Sections stained with caspase-3 antibody at four different time points (DH: A, C, E and G; BS2: B, D, F and H); a higher magnification (I); the growth plate (J) and fibrocartilage of anterior cruciate ligament (K) show caspase-3 positive cells (red cytoplasmic staining; arrow). A negative control section is shown in (L). Image collected and cropped by CiteAb from the following publication (https://www.mdpi.com/1422-0067/14/9/17729), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Caspase-3 by Immunocytochemistry/Immunofluorescence
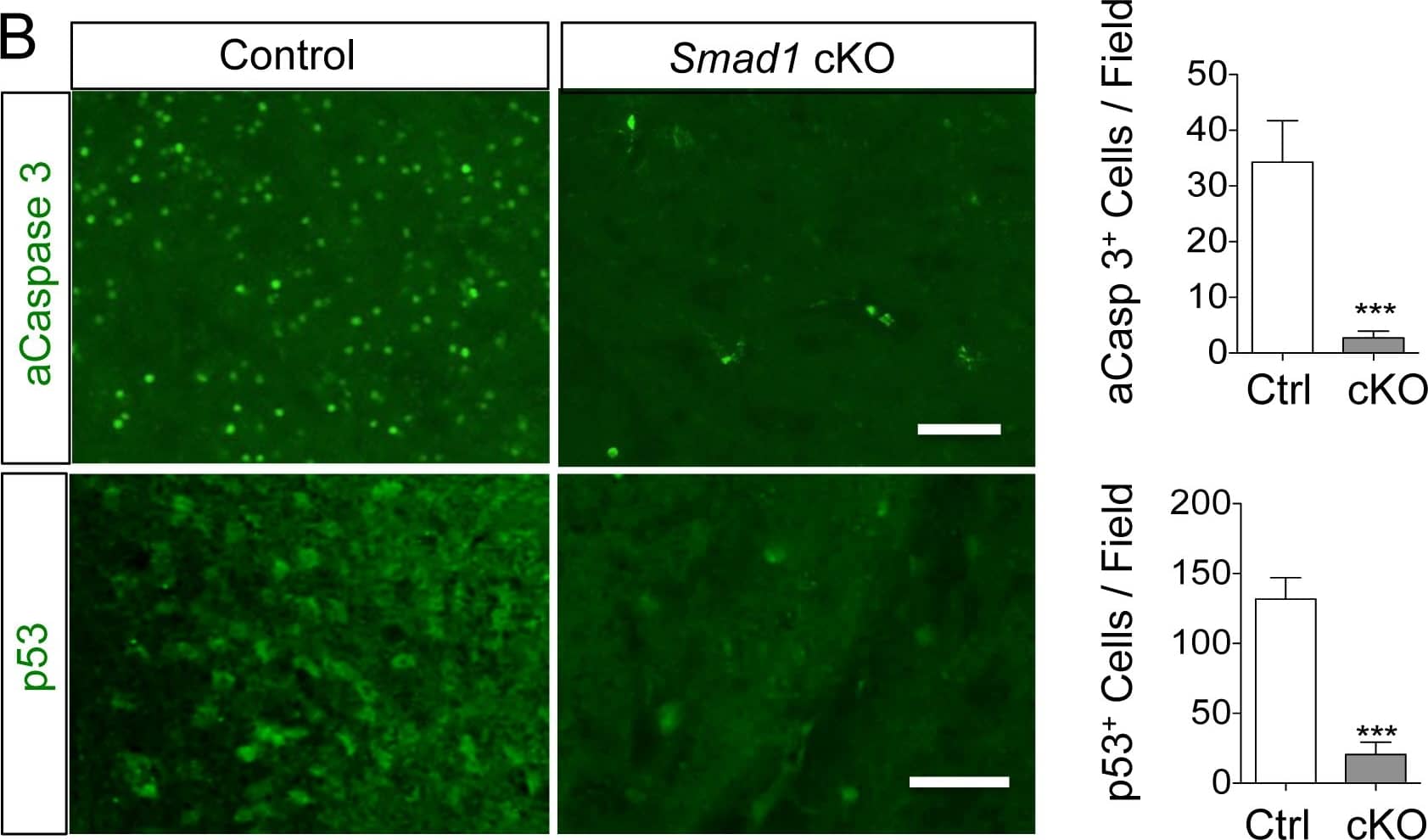
Smad1 deletion reduces oxidative stress and apoptosis following stroke.(A) Fluorescent images and quantification of in vivo HEt assay reflecting ROS levels on the ipsilateral hemisphere at 7 days post-stroke. The extent of oxidized nucleotides was measured by IHC for 8-oxo-dG levels at 24 h post-stroke. (B) IHC and quantification of activated Caspase 3 (aCasp3) and p53 on ipsilateral hemisphere at 7 days post-stroke. n = 3 for HEt, 8-oxo-dG, aCasp3, n = 4 for p53, unpaired Student’s t-test, *p<0.05; ***p<0.001. Scale, 50 μm. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0136967), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Canine Caspase-3 by Western Blot
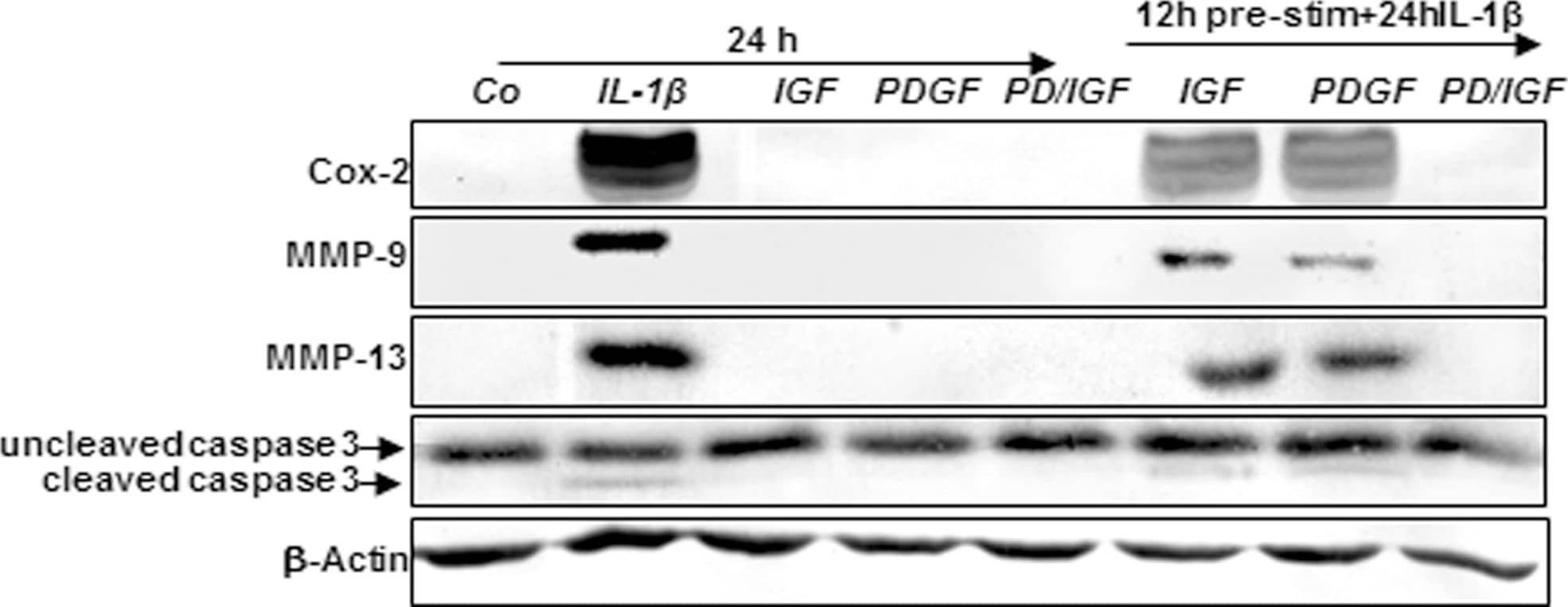
Effects of IGF-1 or/and PDGF-bb on IL-1 beta-induced NF-kappa B-dependent pro-inflammatory, pro-apoptotic and matrix degrading gene products in chondrocytes.To determine whether IGF-1 or/and PDGF-bbexert effects on IL-1 beta-induced NF-kappa B-dependent expression of pro-inflammatory, pro-apoptotic and matrix degrading gene products, primary chondrocytes were either stimulated with 10 ng/ml IL-1 beta, 10 ng/ml PDGF-bb, 10 ng/ml IGF-1 or combination of both growth factors (5 ng/ml each) or pre-stimulated for 12 h with 10 ng/ml PDGF-bb, 10 ng/ml IGF-1 or combination of both growth factors (5 ng/ml each) followed by 10 ng/ml IL-1 beta for 24. Equal amounts of total proteins were separated by SDS-PAGE and analyzed by immunoblotting using antibodies raised against COX-2, MMP-9 and MMP-13 and active caspase-3. Stimulation with IL-1 beta resulted in production of COX-2, MMP-9, MMP-13 and caspase-3 cleavage. Pre-treatment with a combination of both IGF-1 or/and PDGF-bb downregulated COX-2, MMP-9, MMP-13 and cleaved caspase-3. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0028663), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human Human/Mouse Active Caspase-3 Antibody by Immunohistochemistry
Double immunofluorescent staining of FSGS kidneys to Ki-67, alpha-tubulin and DAPI or double immunofluorescent staining to caspase-3 and DAPI. A. FSGS kidneys: cysts with simple squamous epithelium (CS) filled with colloid content, interstitium (i) and collecting tubules (ct) contain Ki-67-positive cells (arrows)(square). B. alpha-tubulin visualizes short cilia (arrows) in the cysts and extremely long cilia in collecting tubules (arrows)(square). C. Nuclear DAPI stain. D. Merging of A + B + C shows relationships between proliferating cells (green) and cilia (red). Immunostaining to Ki-67, alpha-tubulin and DAPI; scale bar 10 μm. E. Distribution of Ki-67 positive cells (%) in CNF and FSGS nephrotic syndrome. Cysts of proximal tubules with simple squamous epithelium (CS) or simple cuboidal epithelium (CC), proximal tubules with apparently normal simple cuboidal/columnar epithelium (PTNC). Data are shown as mean ± SD. Significant differences (Kruskal–Wallis) indicated by ***p < 0.0001. F. FSGS kidneys: caspase-3-positive apoptotic cells (arrows) in proximal tubules (PTNC) and interstitium (i). G. Nuclear DAPI stain. H. Merging of F + G shows green caspase-3- positive apoptotic nuclei and blue non-apoptotic nuclei. Immunostaining to caspase-3 and DAPI, scale bar 10 μm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24397250), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Guinea Pig Human/Mouse Active Caspase-3 Antibody by Immunohistochemistry
Representative sections of the medial side of right tibial epiphysis stained with/without caspase-3. Sections stained with caspase-3 antibody at four different time points (DH: A, C, E and G; BS2: B, D, F and H); a higher magnification (I); the growth plate (J) and fibrocartilage of anterior cruciate ligament (K) show caspase-3 positive cells (red cytoplasmic staining; arrow). A negative control section is shown in (L). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/23994836), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Guinea Pig Human/Mouse Active Caspase-3 Antibody by Immunohistochemistry
Representative sections of the medial side of right tibial epiphysis stained with/without caspase-3. Sections stained with caspase-3 antibody at four different time points (DH: A, C, E and G; BS2: B, D, F and H); a higher magnification (I); the growth plate (J) and fibrocartilage of anterior cruciate ligament (K) show caspase-3 positive cells (red cytoplasmic staining; arrow). A negative control section is shown in (L). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/23994836), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Canine Human/Mouse Active Caspase-3 Antibody by Western Blot
Effects of IGF-1 or/and PDGF-bb on IL-1 beta-induced NF-kappa B-dependent pro-inflammatory, pro-apoptotic and matrix degrading gene products in chondrocytes.To determine whether IGF-1 or/and PDGF-bbexert effects on IL-1 beta-induced NF-kappa B-dependent expression of pro-inflammatory, pro-apoptotic and matrix degrading gene products, primary chondrocytes were either stimulated with 10 ng/ml IL-1 beta, 10 ng/ml PDGF-bb, 10 ng/ml IGF-1 or combination of both growth factors (5 ng/ml each) or pre-stimulated for 12 h with 10 ng/ml PDGF-bb, 10 ng/ml IGF-1 or combination of both growth factors (5 ng/ml each) followed by 10 ng/ml IL-1 beta for 24. Equal amounts of total proteins were separated by SDS-PAGE and analyzed by immunoblotting using antibodies raised against COX-2, MMP-9 and MMP-13 and active caspase-3. Stimulation with IL-1 beta resulted in production of COX-2, MMP-9, MMP-13 and caspase-3 cleavage. Pre-treatment with a combination of both IGF-1 or/and PDGF-bb downregulated COX-2, MMP-9, MMP-13 and cleaved caspase-3. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/22194879), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Caspase-3 by Western Blot
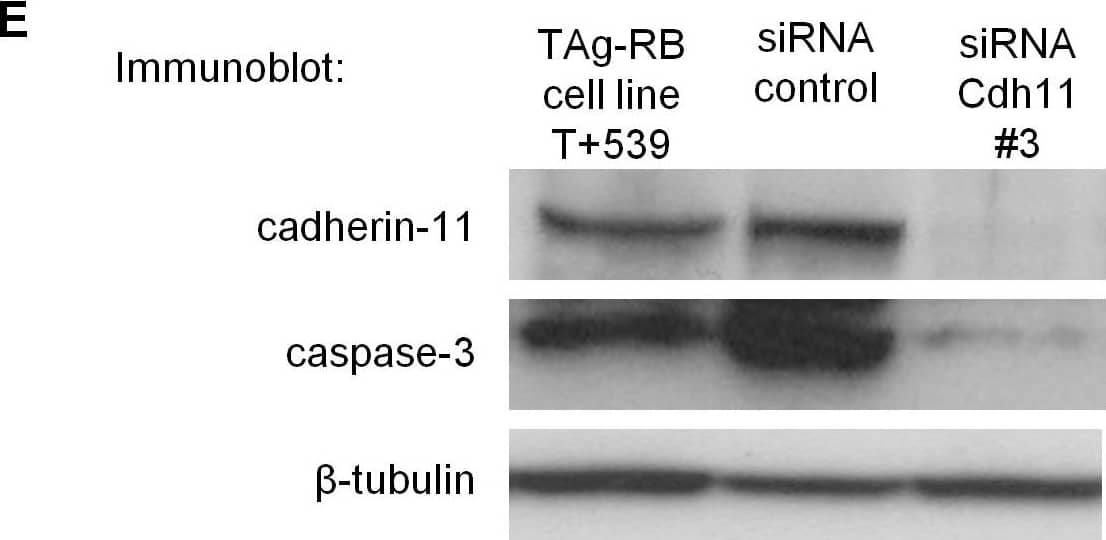
Allelic loss of Cdh11 led to faster growing tumors due to decreased cell death. (E) Cadherin-11 was knocked down using stealth siRNA in a cadherin-11 expressing cell line derived from TAg-RB tumors, T +539. Knockdown of Cdh11 clearly decreased caspase-3 expression compared to control. Image collected & cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/20421947), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Caspase-3 by Western Blot
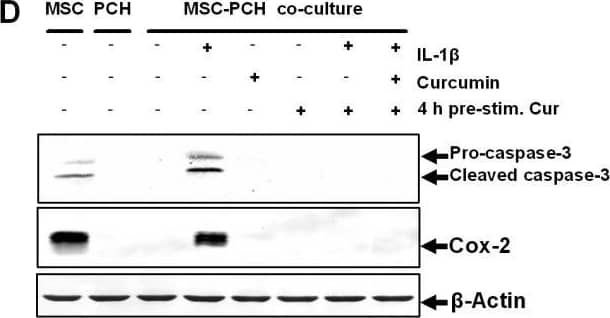
Curcumin inhibits IL-1 beta activity, enabling co-culture induced chondrogenesis in MSCs. A: Fourteen days high-density culture. Untreated MSC cultures became apoptotic (a). In primary chondrocyte cultures (b), co-cultures (c), co-cultures treated with curcumin (e) or co-cultures pre-stimulated four hours with curcumin (f), prominent chondrogenesis was observed. Stimulation of the co-culture with IL-1 beta alone resulted in degeneration of the cell culture (d). In contrast, a four-hour pre-stimulation of the co-culture with curcumin followed by IL-1 beta incubation (g) or a four-hour pre-stimulation of the co-culture with curcumin followed by IL-1 beta and curcumin incubation (h) inhibited the adverse effects of IL-1 beta on the chondrogenic potential of the co-culture and prominent chondrogenesis was observed. Magnification, 6,000×; bar, 1 μm; C, chondrocytes, F, fibroblast-like cells; M, ECM. B-D: Immunoblots of whole cell lysates were probed with antibodies against CSPGs, collagen type II, beta1-integrin, Shc, activated-ERK1/2, Sox-9, activated-caspase-3 and COX-2. In co-cultures pre-stimulated for four hours with curcumin followed either by incubation with IL-1 beta alone or incubation with IL-1 beta and curcumin, prominent production of chondrogenic matrix and adhesion molecules (B), activation of the chondrogenic signalling pathway (C) and down-regulation of apoptotic and inflammatory markers (D) was observed. Each experiment was performed in triplicate. Expression of the housekeeping gene beta-actin was not affected. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/20594343), licensed under a CC-BY license. Not internally tested by R&D Systems.