Human Phospho-Siglec-2/CD22 (Y822) Antibody
R&D Systems, part of Bio-Techne | Catalog # MAB7290

Key Product Details
Validated by
Species Reactivity
Applications
Label
Antibody Source
Product Specifications
Immunogen
Accession # P20273
Specificity
Clonality
Host
Isotype
Scientific Data Images for Human Phospho-Siglec-2/CD22 (Y822) Antibody
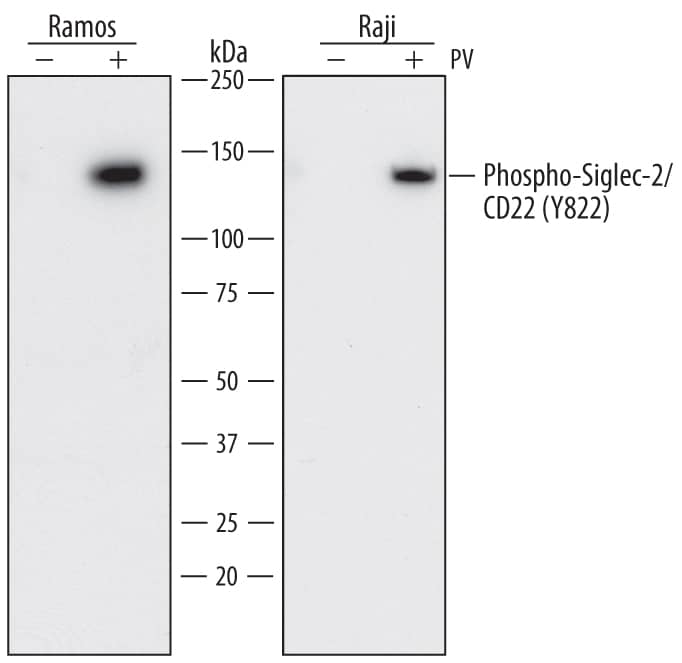
Detection of Human Siglec‑2/CD22 by Western Blot.
Western blot shows lysates of Ramos human Burkitt's lymphoma cell line and Raji human Burkitt's lymphoma cell line untreated (-) or treated (+) with 1 mM Pervanadate (PV) for 30 minutes. PVDF membrane was probed with 1 µg/mL of Mouse Anti-Human Phospho-Siglec-2/CD22 (Y822) Monoclonal Antibody (Catalog # MAB7290) followed by HRP-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # HAF018). A specific band was detected for Siglec-2/CD22 at approximately 140 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.Phospho‑Siglec-2/CD22 (Y822) in Raji Human Cell Line.
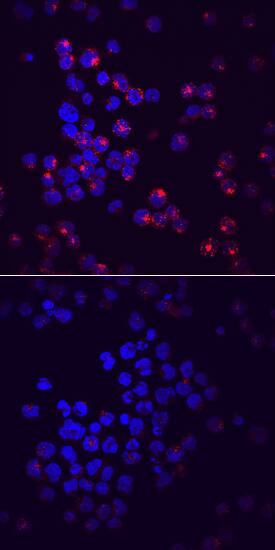
Siglec-2/CD22 phosphorylated at Y822 was detected in immersion fixed Raji human Burkitt's lymphoma cell line unstimulated (lower panel) or stimulated (upper panel) with Pervanadate using Mouse Anti-Human Phospho-Siglec-2/CD22 (Y822) Monoclonal Antibody (Catalog # MAB7290) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Mouse IgG Secondary Antibody (red; Catalog # NL007) and counterstained with DAPI (blue). Specific staining was localized to cytoplasm. View our protocol for Fluorescent ICC Staining of Non-adherent Cells.Applications for Human Phospho-Siglec-2/CD22 (Y822) Antibody
Immunocytochemistry
Sample: Immersion fixed Raji human Burkitt's lymphoma cell line stimulated with PV
Western Blot
Sample: Ramos human Burkitt's lymphoma cell line and Raji human Burkitt's lymphoma cell line treated with Pervanadate (PV)
Formulation, Preparation, and Storage
Purification
Reconstitution
Formulation
Shipping
Stability & Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Siglec-2/CD22
Siglecs (sialic acid binding Ig-like lectins) are I-type (Ig-type) lectins belonging to the Ig superfamily. They are characterized by an N-terminal Ig-like V-type domain which mediates sialic acid binding, followed by varying numbers of Ig-like C2-type domains (1, 2). Eleven human Siglecs have been cloned and characterized. They are sialoadhesin/CD169/Siglec-1, CD22/Siglec-2, CD33/Siglec-3, Myelin-Associated Glycoprotein (MAG/Siglec-4a), and the identified Siglecs 5 to 11 (1-3). To date, no Siglec has been shown to recognize any cell surface ligand other than sialic acid, suggesting that interactions with glycans containing this carbohydrate are important in mediating the biological functions of Siglecs. Human Siglec-2, also known as B-cell antigen CD22 or B lymphocyte cell adhesion molecule (BL-CAM), is a B cell restricted glycoprotein that is expressed in the cytoplasm of progenitor B and pre-B cells and on the surface of mature B cells. Two distinct human Siglec-2/CD22 cDNAs that arise from differential RNA processing of the same gene have been isolated. The predominant Siglec-2/CD22 beta encodes an 847 amino acid (aa) polypeptide with a hydrophobic signal peptide, an N-terminal Ig-like V-type domain, six Ig-like C2-type domains, a transmembrane region and a cytoplasmic tail with 4 immunoreceptor tyrosine-based inhibition motifs (ITIMs) (4). The variant Siglec-2/CD22 alpha encodes a 647 aa polypeptide missing two Ig-like C2-type domains and has a truncated (23 aa) cytoplasmic tail (5). Siglec-2/CD22 is an adhesion molecule that preferentially binds alpha2,6- linked sialic acid on the same (cis) or adjacent (trans) cells. Interaction of CD22 with trans ligands on opposing cells was found to be favored over the binding of ligands in cis (9). Besides its role as an adhesion molecule, Siglec-2/CD22 is a coreceptor that physically interacts with B cell receptor (BCR) and is rapidly phosphorylated upon BCR ligation. It negatively regulates BCR signals by recruiting tyrosine phosphatase SHP-1 to its ITIMs. Phosphorylated Siglec-2/CD22 can also interact with other intracellular effector proteins such as Syk, PLC gamma, PI3 kinase, and Grb-2, suggesting it may play a role in positive signaling (2, 7, 8).
References
- Crocker, P.R. and A. Varki (2001) Trends Immunol. 22:337.
- Crocker, P.R. and A. Varki (2001) Immunology 103:137.
- Angata, T. et al. (2002) J. Biol. Chem. 277:24466.
- Wilson, G.L et al. (1991) J. Exp. Med. 173:137.
- Stamenkovic, I. and B. Seed (1990) Nature 345:74.
- Kelm, S. et al. (1994) Current Bio. 4:965.
- Ravetch, J.V. and L.L. Lanier (2000) Science 290:84.
- Wienands, Y.J. et al. (1999) J. Biol. Chem. 274:18769.
- Collins, B.E. et al. (2004) Proc. Natl. Acad. Sci. USA 101:6104.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional Siglec-2/CD22 Products
Product Documents for Human Phospho-Siglec-2/CD22 (Y822) Antibody
Product Specific Notices for Human Phospho-Siglec-2/CD22 (Y822) Antibody
For research use only