Human Polypeptide GalNAc Transferase 3/GALNT3 Antibody
R&D Systems, part of Bio-Techne | Catalog # AF7174

Key Product Details
Species Reactivity
Validated:
Human
Cited:
Human
Applications
Validated:
Immunocytochemistry, Western Blot
Cited:
Mass Spectrometry, Western Blot
Label
Unconjugated
Antibody Source
Polyclonal Sheep IgG
Product Specifications
Immunogen
Mouse myeloma cell line NS0-derived recombinant human Polypeptide GalNac Transferase 3/GALNT3
Gln38-Asp633
Accession # Q14435
Gln38-Asp633
Accession # Q14435
Specificity
Detects human Polypeptide GalNac Transferase 3/GALNT3 in direct ELISAs and Western blots. In direct ELISAs, less than 1% cross-reactivity with recombinant human (rh) GALNT1 and rhGALNT4 is observed.
Clonality
Polyclonal
Host
Sheep
Isotype
IgG
Scientific Data Images for Human Polypeptide GalNAc Transferase 3/GALNT3 Antibody
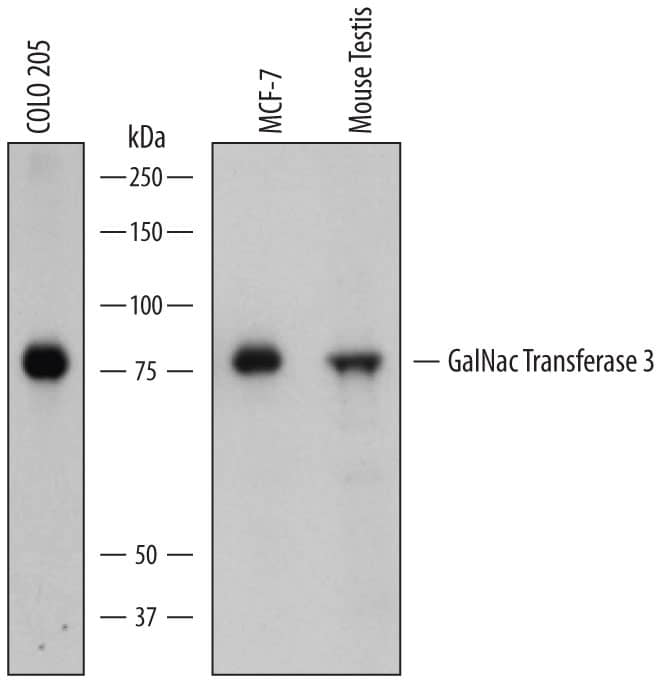
Detection of Human and Mouse Polypeptide GalNac Transferase 3/GALNT3 by Western Blot.
Western blot shows lysates of COLO 205 human colorectal adenocarcinoma cell line, MCF-7 human breast cancer cell line, and mouse testis tissue. PVDF membrane was probed with 0.5 µg/mL of Sheep Anti-Human Polypeptide GalNac Transferase 3/GALNT3 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF7174) followed by HRP-conjugated Anti-Sheep IgG Secondary Antibody (Catalog # HAF016). A specific band was detected for Polypeptide GalNac Transferase 3/GALNT3 at approximately 75 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.Polypeptide GalNac Transferase 3/GALNT3 in HeLa Human Cell Line.
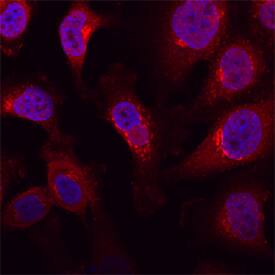
Polypeptide GalNac Transferase 3/GALNT3 was detected in immersion fixed HeLa human cervical epithelial carcinoma cell line using Sheep Anti-Human Polypeptide GalNac Transferase 3/GALNT3 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF7174) at 15 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Sheep IgG Secondary Antibody (red; Catalog # NL010) and counterstained with DAPI (blue). Specific staining was localized to Golgi granules. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.Applications for Human Polypeptide GalNAc Transferase 3/GALNT3 Antibody
Application
Recommended Usage
Immunocytochemistry
5-15 µg/mL
Sample: Immersion fixed HeLa human cervical epithelial carcinoma cell line
Sample: Immersion fixed HeLa human cervical epithelial carcinoma cell line
Western Blot
0.5 µg/mL
Sample: COLO 205 human colorectal adenocarcinoma cell line, MCF‑7 human breast cancer cell line, and mouse testis tissue
Sample: COLO 205 human colorectal adenocarcinoma cell line, MCF‑7 human breast cancer cell line, and mouse testis tissue
Formulation, Preparation, and Storage
Purification
Antigen Affinity-purified
Reconstitution
Sterile PBS to a final concentration of 0.2 mg/mL. For liquid material, refer to CoA for concentration.
Formulation
Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. *Small pack size (SP) is supplied either lyophilized or as a 0.2 µm filtered solution in PBS.
Shipping
Lyophilized product is shipped at ambient temperature. Liquid small pack size (-SP) is shipped with polar packs. Upon receipt, store immediately at the temperature recommended below.
Stability & Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Polypeptide GalNAc Transferase 3/GALNT3
References
- Gerken, T.A. et al. (2011) J. Biol. Chem. 286:14493.
- Ten Hagen, K.G. et al. (2003) Glycobiology 13:1R.
- Hagen, F.K. et al. (1997) J. Biol. Chem. 272:13843.
- Gerken, T.A. et al. (2006) J. Biol. Chem. 281:32403.
- Wandall, H.H. et al. (1997) J. Biol. Chem. 272:23503.
- Pratt, M.R. et al. (2004) Chem. Biol. 11:1009.
- Bennett, E.P. et al. (1996) J. Biol. Chem. 271:17006.
- Wu, Z.L. et al. (2011) Glycobiology 21:727.
Long Name
UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase 3
Alternate Names
GalNAc-T3, HFTC, HHS, pp-GaNTase 3
Gene Symbol
GALNT3
UniProt
Additional Polypeptide GalNAc Transferase 3/GALNT3 Products
Product Specific Notices for Human Polypeptide GalNAc Transferase 3/GALNT3 Antibody
For research use only
Loading...
Loading...
Loading...
Loading...