Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717]
Immunocytochemistry/Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Immunofluorescences against MyHC (red) and Ki67/MKI67 (green) revealing differences in proliferation and differentiation in relation to different PrP concentration. alpha-MEM supplemented with: Ctrl (20% FBS), Low (5 x 10^5 platelets/ml), Medium (1 x 10^6 platelets/ml), and High (1.5 x 10^6 platelets/ml); nuclei were counterstained in blue by DAPI. Scale bars: MyHC = 200 um; Ki67 = 100 um. Image collected and cropped by CiteAb from the following publication (//pubmed.ncbi.nlm.nih.gov/33041857/) licensed under a CC-BY license.
Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717]
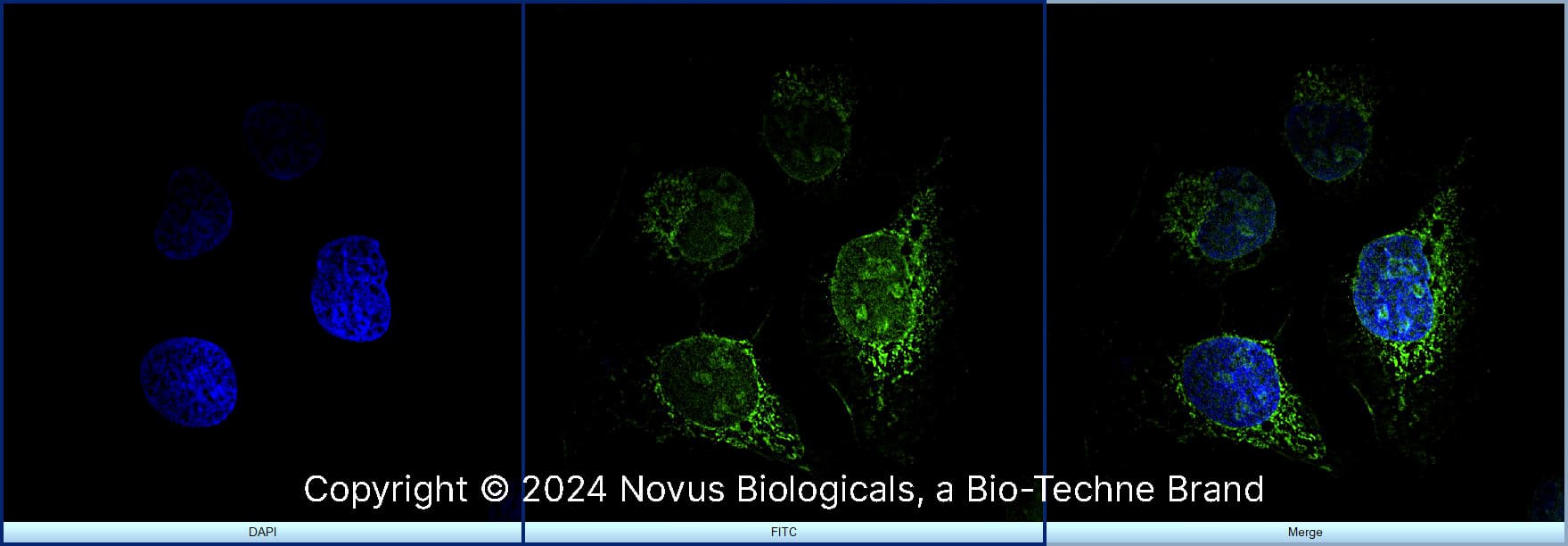
Immunocytochemistry/Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - HeLa cells were fixed for 10 minutes using 10% formalin and then permeabilized for 5 minutes using 1X PBS + 0.5% Triton X-100. The cells were incubated with anti-Ki-67/MKI67 at 2 ug/ml overnight at 4C and detected with an anti-rabbit DyLight 488 (green) at a 1:500 dilution. Nuclei were counterstained with DAPI (blue). Cells were imaged using a 40X objective.
Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717]
Immunocytochemistry/Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - A431 cells were fixed in 4% paraformaldehyde for 10 minutes and permeabilized in 0.5% Triton X-100 in PBS for 5 minutes. The cells were incubated with anti-Ki67/MKI67 Antibody NB110-89717 at 2 ug/ml overnight at 4C and detected with an anti-rabbit Dylight 488 (Green) at a 1:1000 dilution for 60 minutes. Alpha tubulin (DM1A) NB100-690 was used as a co-stain at a 1:1000 dilution and detected with an anti-mouse Dylight 550 (Red) at a 1:1000 dilution. Nuclei were counterstained with DAPI (Blue). Cells were imaged using a 100X objective and digitally deconvolved.
Immunohistochemistry-Paraffin: Ki67/MKI67 Antibody - BSA Free [NB110-89717]
Immunohistochemistry-Paraffin: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Analysis of Ki-67 in paraffin embedded mouse prostate tissue using anti-Ki-67 antibody. Image from verified customer review.
Immunohistochemistry: Ki67/MKI67 Antibody - BSA Free [NB110-89717]
Immunohistochemistry: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Ki-67/MKI67 Antibody [NB110-89717] - Analysis of a mouse intestine cross section. The antibody was used at a dilution of 1:250. Detection: DAB staining. Epitope Retrieval Buffer-High pH was substituted for Epitope Retrieval Buffer-Reduced pH.
Immunohistochemistry-Paraffin: Ki67/MKI67 Antibody - BSA Free [NB110-89717]
Immunohistochemistry-Paraffin: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Staining of a cross section of mouse spleen. Detection: DAB staining using Immunohistochemistry Accessory Kit. Epitope Retrieval Buffer-High pH was substituted for Epitope Retrieval Buffer-Reduced pH.
Immunohistochemistry: Ki67/MKI67 Antibody - BSA Free [NB110-89717]
Immunohistochemistry: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Ki67 Antibody [NB110-89717] - FFPE section of mouse Peyer's patch. Antibody: Affinity purified rabbit anti-mouse Ki-67 used at a dilution of 1:250. Detection: DAB staining using Immunohistochemistry Accessory Kit. Epitope Retrieval Buffer-High pH was substituted for Epitope Retrieval Buffer-Reduced pH.
Immunohistochemistry: Ki67/MKI67 Antibody - BSA Free [NB110-89717]
Immunohistochemistry: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Ki-67/MKI67 Antibody [NB110-89717] - Detection of Ki67 in FFPE mouse intestine using NB110-89717.
Immunohistochemistry-Paraffin: Ki67/MKI67 Antibody - BSA Free [NB110-89717]
Immunohistochemistry-Paraffin: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Analysis of Ki-67 in human prostate xenograft control (left) and treated (right) using anti-Ki-67 antibody. Image from verified customer review.
Flow Cytometry: Ki67/MKI67 Antibody - BSA Free [NB110-89717]
Flow Cytometry: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - An intracellular stain was performed on U-937 cells with NB110-89717PE (blue) and a matched isotype control (orange). Cells were fixed with 4% PFA and then permeabilized with 0.1% saponin. Cells were incubated in an antibody dilution of 2.5 ug/mL for 30 minutes at room temperature. Both antibodies were conjugated to Phycoerythrin.
Flow Cytometry: Ki67/MKI67 Antibody - BSA Free [NB110-89717]
Flow Cytometry: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Ki-67/MKI67 Antibody [NB110-89717] - Staining of mouse bone marrow cells using NB110-89717 at a dilution of 1:100. Photo courtesy of product review by verified customer.
Simple Western: Ki67/MKI67 Antibody - BSA Free [NB110-89717]
Simple Western: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Detection of Ki67/MKI67 by Simple WesternTM. Simple Western lane view shows lysates of HeLa parental cell line and Ki67 knockout (KO) HeLa cell line. A specific band was detected for Ki67/MKI67 at approximately 312 kDa (as indicated) in the parental cell line, but is not detectable in the knockout HeLa cell line using 20 ug/mL of Rabbit Anti-Ki67/MKI67 Polyclonal Antibody (Catalog # NB110-89717). GAPDH is shown as a loading control. This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
Ki67/MKI67 in U-2 OS Human Cell Line.
Ki67/MKI67 was detected in immersion fixed U-2 OS human osteosarcoma cell line using Rabbit anti- Ki67/MKI67 Affinity Purified Polyclonal Antibody conjugated to Alexa Fluor® 488 (Catalog # NB110-89717AF488) (green) at 5 µg/mL overnight at 4C. Cells were counterstained with DAPI (blue). Cells were imaged using a 100X objective and digitally deconvolved.
Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] -
Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Long term Sonidegib treatment in mouse reduces taste buds (TB) & SHH ligand in circumvallate papilla (CV) whereas proliferation & innervation are retained. Immunofluorescent antibody detection of SHH ligand (red) & K8 (green) for TB cells; K8 (red) for TB cells & Ki67 (green) for cell proliferation; & K8 (red) with NF (green) for GL innervation or P2X3 (green) for GL taste fibers, after Vehicle or 48d Sonidegib treatment. For SHH/K8, large dotted lines indicate the basal lamina. Small dotted lines outline the surface epithelium. Inset (K8/P2X3) shows an image of nerves extending into CV epithelial basal lamina (arrow). Scale bar: 50 μm, applies to all images. Inset at 2×. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30385780), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunohistochemistry: Ki67/MKI67 Antibody - BSA Free [NB110-89717] -
Immunohistochemistry: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - KLF4 ablation leads to abnormal proliferation & differentiation in small intestinal epithelium.(A) Small intestine from Klf4−/− mice induced by tamoxifen for different time endurances were stained by H&E & PAS, & also immunohistochemistry staining was performed with anti-Ki67, anti-Lysozyme, anti-DCAMKL-1, & anti-PCNA antibodies respectively. (B) Statistic analysis of IHC staining results from (A). (*, P<0.05) (C) IHC staining from (A) in higher magnification of highlighted frames. Bottom panel: IHC staining with ZO-1 antibody in one-month knockout intestine tissue. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/22384261), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunohistochemistry: Ki67/MKI67 Antibody - BSA Free [NB110-89717] -
Immunohistochemistry: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - KLF4 ablation leads to abnormal proliferation & differentiation in small intestinal epithelium.(A) Small intestine from Klf4−/− mice induced by tamoxifen for different time endurances were stained by H&E & PAS, & also immunohistochemistry staining was performed with anti-Ki67, anti-Lysozyme, anti-DCAMKL-1, & anti-PCNA antibodies respectively. (B) Statistic analysis of IHC staining results from (A). (*, P<0.05) (C) IHC staining from (A) in higher magnification of highlighted frames. Bottom panel: IHC staining with ZO-1 antibody in one-month knockout intestine tissue. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/22384261), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] -
Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Culture conditions influence cell behavior. Immunofluorescences against MyHC (red) & Ki67 (green) reveal the remarkable differences in terms of differentiation & proliferation employing Cyto-Grow commercial medium compared with alpha-MEM (nuclei labeled in blue by DAPI). The respective quantifications are reported as fusion index & rate of proliferating nuclei (Ki67 positive). Statistical analysis: one-way ANOVA & Tukey’s test. **p < 0.01, ***p < 0.001 (n = 3). Scale bars: MyHC = 200 μm; Ki67 = 100 μm. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/33041857), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] -
Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Paclitaxel in combination with MWE retarded tumor growth in a human bladder carcinoma TSGH 8301 xenograft model.(a) TSGH 8301 cells (1 × 107 cells/mouse) were injected into the right inguinal region of a nude mouse to form tumor xenografts. When the tumor size reached approximately 250 to 300 mm3, the mice were randomly divided into 4 groups & received the following treatments: paclitaxel combined with MWE, MWE alone, paclitaxel alone & sterile deionized water (control group). Tumor size was monitored every week, & the results are expressed as the percentage of the size at week 0 (the day treatment started) for each group. (b) The levels of total (t-PTEN) & phospho-PTEN (p-PTEN) & Caspase 3 in the tumor specimens were determined by Western blotting & then quantified using beta-actin as the protein loading control; the results are expressed as a percentage of the control. (c) Immunohistochemical examination of p-PTEN in the tumor sections obtained from the indicated treatment. (d) & (e) Fluorescent immunohistochemical detection of Ki67 & TUNEL examination in the xenografts obtained from the indicated treatment. (f ) Western blotting analysis of the levels of Cyclin B1, Cdc2 & Aurora A in xenograft tumors. Arrow indicates the Ki67 or TUNEL positive cells. One-way ANOVA with post-hoc Dunnett’s test was used to calculate the p value for each treatment compared to paclitaxel alone, (+p < 0.05;++p < 0.01) at each time point (**indicates p < 0.01 & *indicates p < 0.05). Image collected & cropped by CiteAb from the following publication (https://www.nature.com/articles/srep20417), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] -
Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Sonidegib treatment reduces taste buds (TB), SHH ligand & proliferation in rat fungiform papilla (FP) while innervation is retained. (a) Immunofluorescent antibody detection of SHH ligand (red) & K19 (green) for TB cells; K18 (red) for TB cells & Ki67 (green) for cell proliferation, after Vehicle, 16d or 28d Sonidegib treatments. SHH is reduced in association with TB, K19+ cell loss. Asterisks (*) indicate nonspecific SHH immunoproduct in cornified surface cells in 16d Sonidegib image. The Vehicle, K18/Ki67 image shows 3 regions positive for Ki67+ cells (Apical, Basal & Perigemmal). Proliferating cells are lost in Apical FP region after 16–28d Sonidegib. (b) Number of Ki67+ cells in Apical & Basal regions of FP in Vehicle- & Sonidegib-treated mice. Numbers of tongues analyzed are in parentheses. For each tongue 8–10 FP were analyzed. Sonidegib treatment reduces apical epithelial cell proliferation in FP compared to Vehicle. Statistical analysis was one-way ANOVA with Tukey HSD posthoc comparisons (*p ≤ 0.05, compared to Vehicle, APICAL). (c) Immunofluorescent antibody detection of K19 or K18 (red) for TB cells & NF (green) for lingual & CT innervation or P2X3 (green) for CT nerve fibers. Innervation was retained after Sonidegib exposure. Asterisks (*) indicate nonspecific P2X3 immunoproduct in surface layer in Vehicle image. (d) Enlarged images from 28d Sonidegib papillae. Arrows point to NF+ or P2X3+ fibers in the FP epithelium. Throughout, white dotted lines indicate the basal lamina. Yellow dotted lines indicate surface of epithelium. (a,c) Scale bar: 50 μm, applies to all images. (d) Scale bar: 25 μm, applies to both images. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30385780), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] -
Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - SPIN1 controls proliferation & apoptosis of liposarcoma cell-derived tumors in BALB/c nude mice(A) Analysis of tumors from BALB/c nude mice (n = 15) 10 days after subcutaneous injection of T778 cells expressing control miRNA (miCtrl) or miRNA against SPIN1 [miSPIN1(1)]. Scale bar = 5 mm. (B) Average tumor weight of mice shown in (A) (C) Quantitative RT-PCR analysis of SPIN1, GDNF, & RET expression in T778 cell-derived tumors treated with the indicated miRNA. (D) Western blot analysis of SPIN1, RET, & RETph levels in T778 cell-derived tumors treated with the indicated miRNA. alpha-Tubulin & GFP were used as loading controls. (E) Detection of Ki67 by immunofluorescence in T778 cell-derived tumors treated with the indicated miRNA. Scale bar = 100 μm. (F) Quantification of Ki67 staining shown in (E). (G) TUNEL assay for detection of apoptotic cells in T778 cell-derived tumors treated with the indicated miRNA. Scale bar = 100 μm. (H) Quantification of TUNEL staining shown in (G) (B, C, F, H) Error bars represent +/– SEM, *p < 0.05, **p < 0.01. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/25749382), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] -
Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Sonidegib treatment in rat reduces taste buds (TB) & SHH ligand in circumvallate papilla (CV) whereas cell proliferation & GL innervation are retained. Immunofluorescent antibody detection of SHH ligand (red) & K19 (green) for TB cells; K18 (red) for TB cells & Ki67 (green) for cell proliferation; K19 (red) for TB cells & NF (green) for innervation; and, K18 (red) for TB cells & P2X3 (green) for taste nerve fibers, after Vehicle or 36d Sonidegib treatment. White dotted lines outline the epithelium. Asterisk in SHH/K19, 36d Sonidegib indicates nonspecific K19 immunostaining. Arrow points to the P2X3+ nerves extending into CV epithelium after Sonidegib treatment. SHH is reduced in association with TB cells. Cell proliferation is maintained & nerves fibers are retained after Sonidegib treatment. Scale bar: 50μm, applies to all images, except K19/NF. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30385780), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] -
Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Overexpression of Chibby expression inhibits cell proliferation & invasion of HCC cells. (A) Adenovirus-mediated ectopic expression of Chibby & enhanced the expression of Chibby in Huh7 cells. After 48 h of infection with adenoviral vectors (Ad-GFP or Ad-Chibby) at different multiplicity of infection, the protein lysates from the Huh7 cells were harvested to determine the ectopic gene expression using Western blot analysis. Ad-GFP was designed as the control vector. (B) Representative immunofluorescent images of Ki67 in Huh7-normal control, Huh7-Ad-GFP, & Huh7-Ad-Chibby cells. (C,D) The enhancement of Chibby in Huh7 cells transfected with Ad-Chibby suppressed cell proliferation & invasiveness by colon-formation assay & the Boyden chamber system, respectively. Data represent mean ± SE from three independent analyses. Scale bar, 100 μm, * p < 0.05 & ** p < 0.01 vs. Ad-GFP group. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32192213), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] -
Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - High doses of metformin inhibit the proliferation of C2C12 cells without inducing apoptosis.(a) C2C12 cells were treated with different doses of metformin in growth medium (GM) & the total number of cells was counted after 1, 2, 3 & 4 days of treatment by immunofluorescence microsopy. The initial number of plated cells was the same in each growth condition. Statistical significance was evaluated by the Student’s t-test (*p<0.05) (b) TUNEL assay. C2C12 cells were treated with 100μM, 2mM & 10mM metformin for 48h. As positive control for the TUNEL assay C2C12 myoblasts were incubated with DNΑse I before staining and, as negative control, cells were stained with the label solution without the addition of the reaction enzyme terminal deoxynucleotidyl transferase (TdT). (c) Total protein extracts of C2C12 myoblasts treated with 100μM, 2mM & 10mM were analyzed by SDS-PAGE for the expression of the apoptotic markers cl-caspase 3 & cl-caspase 7. For the induction of apoptosis in the positive control was used staurosporine 1μM for 4 hours. GAPDH is used as loading control (d) Proliferating C2C12 myoblasts, were plated at the same initial number (4*104 in GM in 9,5 cm2 area wells), incubated with 100μM, 2mM & 10mM metformin for 48h. The percentage of cells expressing Ki67 was measured by Cell Profiler cell image analysis software. Statistical significance was evaluated by the Student’s t-test (*p<0.05). Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28859084), licensed under a CC-BY license. Not internally tested by Novus Biologicals.

![Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717]](https://resources.bio-techne.com/images/products/Ki67-MKI67-Antibody---BSA-Free-Immunocytochemistry-Immunofluorescence-NB110-89717-img0023.jpg)
![Immunohistochemistry: Ki67/MKI67 Antibody - BSA Free [NB110-89717] Immunohistochemistry: Ki67/MKI67 Antibody - BSA Free [NB110-89717]](https://resources.bio-techne.com/images/products/Ki67-MKI67-Antibody---BSA-Free-Immunohistochemistry-NB110-89717-img0020.jpg)
![Flow Cytometry: Ki67/MKI67 Antibody - BSA Free [NB110-89717] Flow Cytometry: Ki67/MKI67 Antibody - BSA Free [NB110-89717]](https://resources.bio-techne.com/images/products/Ki67-MKI67-Antibody---BSA-Free-Flow-Cytometry-NB110-89717-img0017.jpg)
![Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717]](https://resources.bio-techne.com/images/products/Ki67-MKI67 Antibody - BSA Free-Immunocytochemistry-Immunofluorescence-NB110-89717-img0024.jpg)
![Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717]](https://resources.bio-techne.com/images/products/Ki67-MKI67-Antibody---BSA-Free-Immunocytochemistry-Immunofluorescence-NB110-89717-img0015.jpg)
![Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717]](https://resources.bio-techne.com/images/products/Ki67-MKI67-Antibody---BSA-Free-Immunocytochemistry-Immunofluorescence-NB110-89717-img0022.jpg)
![Immunohistochemistry-Paraffin: Ki67/MKI67 Antibody - BSA Free [NB110-89717] Immunohistochemistry-Paraffin: Ki67/MKI67 Antibody - BSA Free [NB110-89717]](https://resources.bio-techne.com/images/products/Ki67-MKI67-Antibody---BSA-Free-Immunohistochemistry-Paraffin-NB110-89717-img0009.jpg)
![Immunohistochemistry: Ki67/MKI67 Antibody - BSA Free [NB110-89717] Immunohistochemistry: Ki67/MKI67 Antibody - BSA Free [NB110-89717]](https://resources.bio-techne.com/images/products/Ki67-MKI67-Antibody---BSA-Free-Immunohistochemistry-NB110-89717-img0010.jpg)
![Immunohistochemistry-Paraffin: Ki67/MKI67 Antibody - BSA Free [NB110-89717] Immunohistochemistry-Paraffin: Ki67/MKI67 Antibody - BSA Free [NB110-89717]](https://resources.bio-techne.com/images/products/Ki67-MKI67-Antibody---BSA-Free-Immunohistochemistry-Paraffin-NB110-89717-img0012.jpg)
![Immunohistochemistry: Ki67/MKI67 Antibody - BSA Free [NB110-89717] Immunohistochemistry: Ki67/MKI67 Antibody - BSA Free [NB110-89717]](https://resources.bio-techne.com/images/products/Ki67-MKI67-Antibody---BSA-Free-Immunohistochemistry-NB110-89717-img0014.jpg)
![Immunohistochemistry: Ki67/MKI67 Antibody - BSA Free [NB110-89717] Immunohistochemistry: Ki67/MKI67 Antibody - BSA Free [NB110-89717]](https://resources.bio-techne.com/images/products/Ki67-MKI67-Antibody---BSA-Free-Immunohistochemistry-NB110-89717-img0006.jpg)
![Immunohistochemistry-Paraffin: Ki67/MKI67 Antibody - BSA Free [NB110-89717] Immunohistochemistry-Paraffin: Ki67/MKI67 Antibody - BSA Free [NB110-89717]](https://resources.bio-techne.com/images/products/Ki67-MKI67-Antibody---BSA-Free-Immunohistochemistry-Paraffin-NB110-89717-img0019.jpg)
![Flow Cytometry: Ki67/MKI67 Antibody - BSA Free [NB110-89717] Flow Cytometry: Ki67/MKI67 Antibody - BSA Free [NB110-89717]](https://resources.bio-techne.com/images/products/Ki67-MKI67-Antibody---BSA-Free-Flow-Cytometry-NB110-89717-img0016.jpg)
![Flow Cytometry: Ki67/MKI67 Antibody - BSA Free [NB110-89717] Flow Cytometry: Ki67/MKI67 Antibody - BSA Free [NB110-89717]](https://resources.bio-techne.com/images/products/Ki67-MKI67-Antibody---BSA-Free-Flow-Cytometry-NB110-89717-img0004.jpg)
![Simple Western: Ki67/MKI67 Antibody - BSA Free [NB110-89717] Knockout Validated: Ki67/MKI67 Antibody - BSA Free [NB110-89717]](https://resources.bio-techne.com/images/products/Ki67-MKI67-Antibody---BSA-Free-Knockout-Validated-NB110-89717-img0021.jpg)
![Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Ki67/MKI67 Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb110-89717_rabbit-polyclonal-ki67-mki67-antibody-310202415175296.jpg)
![Immunohistochemistry: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Ki67/MKI67 Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb110-89717_rabbit-polyclonal-ki67-mki67-antibody-310202415394542.jpg)
![Immunohistochemistry: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Ki67/MKI67 Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb110-89717_rabbit-polyclonal-ki67-mki67-antibody-310202415175256.jpg)
![Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Ki67/MKI67 Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb110-89717_rabbit-polyclonal-ki67-mki67-antibody-310202415284548.jpg)
![Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Ki67/MKI67 Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb110-89717_rabbit-polyclonal-ki67-mki67-antibody-31020241621846.jpg)
![Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Ki67/MKI67 Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb110-89717_rabbit-polyclonal-ki67-mki67-antibody-310202416205161.jpg)
![Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Ki67/MKI67 Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb110-89717_rabbit-polyclonal-ki67-mki67-antibody-31020241620513.jpg)
![Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Ki67/MKI67 Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb110-89717_rabbit-polyclonal-ki67-mki67-antibody-31020241621233.jpg)
![Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Ki67/MKI67 Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb110-89717_rabbit-polyclonal-ki67-mki67-antibody-310202416212380.jpg)
![Immunocytochemistry/ Immunofluorescence: Ki67/MKI67 Antibody - BSA Free [NB110-89717] - Ki67/MKI67 Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb110-89717_rabbit-polyclonal-ki67-mki67-antibody-310202416212356.jpg)