Western Blot Analysis of LC3B in Treated U87-MG Lysates
Analysis of LC3B in treated U87-MG (human glioblastoma astrocytoma) lysates using anti- [Catalog # NB600-1384].
Western Blotting of LC3B in Treated Control and RARalpha-Knockdown SKBR3 Cells
LC3B-Antibody---BSA-Free-Western-Blot-NB600-1384-img0040.jpg
Detection of LC3B in Treated and Untreated HeLa and Nero2a Cells in Western Blot
Human cervical carcinoma (HeLa) and Mouse Neuroblast cells (Neuro2a) were treated with (+) and without (-) 50 uM Chloroquine overnight. Whole cell protein lysates were prepared in 1x Laemmli sample buffer and approximately 10 ug of each lysate (NBP2-49689 and NBP2-49688) was separated on a 4- 20% gel by SDS-PAGE, transferred to 0.2 um PVDF membrane and blocked in 5% nonfat milk in TBST. The membrane was probed with 2 ug/mL anti-LC3 (NB600-1384) and 1 ug/mL anti-alpha tubulin (NB100-690) as a loading control, and detected with the appropriate secondary antibodies using NovaLume Pico Chemiluminescence Substrate (NBP2-61915).
Immunocytochemistry/Immunofluorescence Staining of LC3B in Treated Fibroblasts
LC3B Antibody - BSA Free-Immunocytochemistry-Immunofluorescence-NB600-1384-img0045.jpg
Immunocytochemistry/Immunofluorescence Detection of LC3B in HeLa Cells
Confocal analysis of HeLa cells using Rabbit anti-LC3B antibody (Catalog # NB600-1384, 1:5). An Alexa Fluor 488-conjugated Goat to rabbit IgG was used as secondary antibody (green). Actin filaments were labeled with Alexa Fluor 568 phalloidin (red). DAPI was used to stain the cell nuclei (blue).
Staining of LC3B in Treated U373-MG Cells Using HRP Conjugated LC3B Antibody
Immunocytochemical/Immunofluorescent staining of treated U373-MG cells using the HRP conjugate of anti- (Catalog # NB600-1384). The nuclei were stained with DAPI.
Immunocytochemical Staining of LC3B in Treated U373-MG Cells
Staining of treated U373-MG (human glioblastoma) cells using anti- [Catalog # NB600-1384].
Immunohistochemical Staining of LC3B in Paraffin Embedded U87MG Glioma Xenografts
Analysis of U87MG glioma xenografts using anti- [Catalog # NB600-1384]. Image from verified customer review.
Immunohistochemical Staining of LC3B in Treated U87-MG Cultured and Subcutaneous Tumors
LC3B staining in treated U87-MG cultured & subcutaneous tumors.
Immunohistochemical Staining of LC3B in Glioblastoma Multiform Tissue
LC3B staining in glioblastoma multiform tissue.
Simple Western Analysis of LC3B in Neuro2A Lysate
Lane view shows a specific band for LC3B in 0.5 mg/ml of Neuro2A lysate at a molecular weight of approximately 15 kDa. This experiment was performed under reducing conditions using the 12-230 kDa separation system.
Knockdown Validation of LC3B Antibody in Multiple Applications
LC3B-Antibody---BSA-Free-Knockdown-Validated-NB600-1384-img0034.jpg
Detection of LC3B in Infected Human Alveolar Macrophages Using Electron Microscopy
LC3B-Antibody---BSA-Free-Electron-Microscopy-NB600-1384-img0038.jpg
Immunohistochemistry: LC3B Antibody - BSA Free [NB600-1384] -
Examples of immunohistochemical stainings; (A) LC3B low, (B) LC3B high, (C) p62 low (any cellular compartment), (D) p62 cytoplasmic low, (E) p62 cytoplasmic/dot like high, nuclear low, (F) p62 cytoplasmic/dot like low, nuclear high, (G) HMGB1 low, (H) HMGB1 high. Objective magnification, 40×.
Simple Western: LC3B Antibody - BSA Free [NB600-1384] -
Simple Western: LC3B Antibody - BSA Free [NB600-1384] - Autolysosome imaging after growth factor deprivation captured the autophagy induction in iPSC-derived astrocytes. (A) Schematic procedures of autophagy induction assay. (B) Representative images of cells and autolysosomes on day 2 with or without growth factor deprivation. (C) Fluorescence counts per well of 96-well plate at 16 h after initiation of assay. Astrocytes were treated with control, starvation and starvation with 10 nM bafilomycin A1 (BafA1) condition. The data were analysed by one-way ANOVA (F [3, 12] = 21.19, p < 0.0001), followed by Tukey's multiple comparisons test. *p < 0.05, **p < 0.01, ****p < 0.001, ns, not significant. Data represent mean +- SD (n = 4, from different experimental replicates). (D) Astrocytes were treated with control, starvation and starvation with 10 nM bafilomycin A1 (BafA1) condition. Cells were lysed and subjected to immunoblot analysis at 16 h after initiation of assay. M.W., molecular-weight size marker. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/38509731 ), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunocytochemistry/ Immunofluorescence: LC3B Antibody - BSA Free [NB600-1384] -
Autophagy associated protein immunoreactivity in HIV-infected brain tissue. (A) Representative images from five randomly selected fields of cells each examined in duplicate frontal lobe white matter sections for the indicated subject groups. The indicated proteins were labeled red & microglia with the cell-type-specific marker Iba1 (green). Blue staining indicates cell nuclei. Arrow heads indicate examples of higher Iba1 immunoreactivity whereas arrows indicate more focal (punctal) vs. diffuse (filamentous) patterns of autophagy associated protein expression. Scale bar = 10 μm. (B) Quantification of relative Iba1 immunoreactivity from (A). F(3,20) = 6.450, p = 0.0031; ∗p < 0.05 when compared to all other subject groups. Error bars show the SEM for the average values of 2–6 regions from each subject group across the six autophagy associated proteins examined. (C) Quantification of the indicated autophagy associated protein relative immunoreactivity from (A). Beclin 1: F(3,12) = 11.29, p = 0.0008; LC3B: F(3,12) = 1.994, p = 0.1687; APG7/ATG7: F(3,12) = 84.20, p = < 0.0001; ATG5: F(3,12) = 6.218, p = 0.0086; p62/SQSTM1: F(3,12) = 87.04, p = < 0.0001; LAMP1: F(3,12) = 8.317, p = 0.0029. ∗p < 0.05 when compared to HIV-negative; #p < 0.05 when compared to HIV-positive; & Ωp < 0.05 when compared to HIV-positive/NCI subjects. Error bars show the SEM for four regions from each subject group. Image collected & cropped by CiteAb from the following publication (http://journal.frontiersin.org/Article/10.3389/fmicb.2015.00653/abstract), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Fucoxanthin failed to suppress oxidative stress & activate autophagy in Nrf2−/− mice following TBI.Fucoxanthin treatment had no effect on change the level of MDA & the activity of GPx (A) & the expression of Beclin-1, LC3 & p62 (B) in Nrf2−/− mice compared to the vehicle-treated group. n = 6 per group. @p > 0.05 versus TBI + vehicle group. beta-actin was used as a loading control. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28429775), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Differences in neuronal autophagy & dendrite varicosity following HIV-1 Tat protein & morphine treatment. (A) Representative images of neurons transfected with a fluorescent reporter plasmid to monitor autophagic flux at 8 h following the indicated treatments. GFP (green) & GFP + mRFP (yellow) fluorescence are observed prior to the fusion of autophagosomes with lysosomes whereas only mRFP (red) fluorescence is present in post-fusion autolysosomes. DIC, differential interference contrast microscopy image. DAPI (blue) staining indicates cell nuclei. (B) Quantification of autolysosomes (red puncta) from (A). F(3,13) = 8.756, p = 0.0019; ∗p < 0.05 when compared to all other groups. (C) Western blotting analysis of the indicated autophagy associated protein levels at 24 h following the indicated treatments. GAPDH was used as a loading control. Blots are representative of three independent experiments. (D) Quantification of dendrite beading from (A). F(3,77) = 6.429, p = 0.0006; ∗p < 0.05 when compared to control cells. Error bars show the SEM. Image collected & cropped by CiteAb from the following publication (http://journal.frontiersin.org/Article/10.3389/fmicb.2015.00653/abstract), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
HMGB1 regulated autophagy in thyroid cancer cells (a) FTC-133/TPC-1 cells were transfected with HMGB1 shRNA & control shRNA & then starved by HBSS for 2 h. & LC3-I/II level was assayed by Western blot; (b) FTC-133/TPC-1 cells were transfected with HMGB1 shRNA & control shRNA & then pre-treated for 1 h with pepstatin A (PA, 10 μM) & E64D (10 μM) as indicated. Cells were subsequently treated for 3 h with HBSS in continuous presence or absence PA/E64D inhibitors. LC3-I/II, Beclin1 & p62 levels were assayed by Western blot; (c) Ultrastructural features in FTC-133/TPC-1 cells transfected with HMGB1 shRNA & control shRNA after a 3-h treatment of HBSS. More autophagosomes were seen in control shRNA plus HBSS-treated cells than in cells treated with HMGB1 shRNA plus HBSS. Arrows indicated autophagosomes Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31331356), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Fucoxanthin activated autophagy after TBI.(A) Representative images of immunofluorescence for LC3 surrounding the injured cortex. LC3 punctate dots were observed in the cytoplasm by immunofluorescent staining of LC3 (red). Neuron cells & nuclei are labeled with NeuN (green) & DAPI (blue), respectively. Magnification: 40 x. Scale bar: 50 mm. (B) Mice brain tissues were collected 1 day after TBI in different groups, & the expression of LC3, Beclin-1 & p62 was measured by western blot. Fucoxanthin treatment significantly increased the level of LC3-II & Beclin-1 while decreasing the level of p62 after TBI. (C) 3-MA (400 nM) was injected i.c.v. 30 min before TBI. Mice were then subjected to TBI & treatment of fucoxanthin 30 min after TBI. Pretreatment with 3-MA significantly attenuated fucoxanthin-induced activation of autophagy & suppression of apoptosis & oxidative stress in the ipsilateral cortex. Data are presented as mean ± SEM, n = 6 per group; **p < 0.01, ***p < 0.001 versus sham group; #p < 0.05, ##p < 0.01 versus TBI + vehicle group; &&p < 0.01, &&&p < 0.001 versus TBI + fucoxanthin group. beta-actin was used as a loading control. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28429775), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Fucoxanthin protected primary cultured neurons from TBI.(A) Primary cortical neurons were subjected to scratch injury & then treated with 5, 10 or 20 μM fucoxanthin or DMSO for 1 day. The LDH release assay was used to evaluate cell viability. The percentage of survival cells significantly decreased after TBI compared to the control group. Fucoxanthin treatment significantly increased survival cells after TBI. (B) Fucoxanthin repressed the production of ROS in primary cultured cells after TBI. Cells were subjected to scratch injury & subsequently treated with 100 μM edaravone or 5, 10 or 20 μM fucoxanthin or DMSO for 1 day. Then cells were incubated with DCFH-DA & subjected to fluorescence spectrophotometer analysis. The intracellular ROS was significantly increased after TBI compared to the sham group, & administration of edaravone or fucoxanthin significantly repressed ROS production as compared to the TBI + DMSO group. (C) Fucoxanthin inhibited apoptosis & activated autophagy in primary cultured neurons. Primary cortical neurons were subjected to scratch injury & then treated with 5, 10 or 20 μM fucoxanthin or DMSO for 1 day, the expression of cleaved caspase-3, Beclin-1, LC3 & p62 was measured by western blot. Fucoxanthin significantly decreased the expression of cleaved caspase-3 & p62 while increased the expression of Beclin-1 & LC3-II. Data are presented as mean ± SEM, n = 6 per group; *p < 0.05, **p < 0.01, ***p < 0.001 versus control group; #p < 0.05, ##p < 0.01, ###p < 0.001 versus TBI + DMSO group; @p > 0.05 versus TBI + DMSO group. beta-actin was used as a loading control. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28429775), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
8-Cl-Ado-induces autophagic cell killing. (A) Western blot analysis of beclin1 & ATG7 levels in MCF-7 cells transfected with either a pool of control siRNA (siCONT), siRNA targeting the expression of the beclin1 gene (siBECN1), or targeting the expression of the ATG7 gene (siATG7). Immunoblot analysis of LC3B lipidation & PARP cleavage were assessed as markers of autophagosome formation & apoptosis, respectively. GAPDH was used as loading control. Flow cytometric analysis of cells transfected with siCONT, solid bars, siBECN1, hatched bars, or siATG7, checkered bars, treated with 10 μM 8-Cl-Ado & stained with (B) annexin V & PI, as well as (C) acridine orange. Effect of autophagy on 8-Cl-Ado-inhibiton of clonogenic survival. Cells transfected with (D) siCONT, ○, or siBECN1, ●, & with (E) siCONT, ○, or siATG7, ●, were treated with the indicated doses of 8-Cl-Ado for 3 days, washed with PBS, & cultured in fresh medium for 10 days. Colonies of >50 cells were counted under a dissecting microscope. Image collected & cropped by CiteAb from the following publication (https://jhoonline.biomedcentral.com/articles/10.1186/1756-8722-7-23), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunocytochemistry/ Immunofluorescence: LC3B Antibody - BSA Free [NB600-1384] -
Immunocytochemistry/ Immunofluorescence: LC3B Antibody - BSA Free [NB600-1384] - Assesment of functional basal autophagy in vitro(A) Immunoblotting of LC3B & p62 upon pharmacological autophagy inhibition. A dose-dependent increase of LC3B & p62 was observed with increasing concentrations of chloroquine. (B) Immunofluorescence of LC3B & p62 upon pharmacological autophagy inhibition. I. In CC cell lines HT-29 & II. LoVo a dose-dependent increase of LC3B & p62 was observed with increasing concentrations of chloroquine. (C) Electron microscopy of CC cell lines pharmacological autophagy inhibition. I-III. In CC cell line HT-29 a dose-dependent increase of cytoplasmic vesicles was observed with increasing concentrations of CQ. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28903368), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - Phosphorylation of AKT & mTOR decreases after treatment with gemcitabine (GCB)A LM7, CCH-OS-D, & K7M3 osteosarcoma cells were treated with 1μM GCB & cell lysates were analyzed by immunoblotting with specific antibodies for AKT, mTOR, p-AKT, & p-mTOR. beta-actin was used as a loading control. Densitometry was performed using ImageJ. B. & C. Following treatment with GCB, lysates were analyzed using the Human Phospho Kinase Antibody Array. The phosphorylation levels of AKT, mTOR, PRAS40, p70S6K, JNK, AMPK, & ERK were evaluated. Densitometry was performed using Image J. Values were graphed as relative fold change. Means ± standard deviation of three independent experiments are shown. Image collected & cropped by CiteAb from the following publication (https://www.oncotarget.com/lookup/doi/10.18632/oncotarget.20308), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - Autophagy decreases during striatal development. (A) Representative Western blot images for LC3B, p62, DARPP32, & actin. Quantification of (B) LC3B-ii relative to actin, (C) LC3B-ii relative to LC3B-i, (D) p62 relative to actin normalized to P120 values. Data analyzed with one-way ANOVA; (B) Age: F(4,21) = 6.526, p = 0.0014; (C) Age: F(4,22) = 3.797, p = 0.0171; (D) Age: F(4,15) = 3.762, p = 0.0260. *p < 0.05, **p < 0.01, n = 4–6 mice/age. (E,F) Representative images of DARPP32 stained striatal neurons & RFP fluorescence from mice aged P10 & P28. Dashed lines indicate cell body outlines. (G) Quantification of number of LC3 puncta/cell. Unpaired, two-tailed t-test, t4 = 4.392, p = 0.0118. N = 3 mice/age, 20–50 cells were analyzed per mouse. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32296308), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunohistochemistry: LC3B Antibody - BSA Free [NB600-1384] -
Immunohistochemistry: LC3B Antibody - BSA Free [NB600-1384] - Examples of immunohistochemical stainings; (A) LC3B low, (B) LC3B high, (C) p62 low (any cellular compartment), (D) p62 cytoplasmic low, (E) p62 cytoplasmic/dot like high, nuclear low, (F) p62 cytoplasmic/dot like low, nuclear high, (G) HMGB1 low, (H) HMGB1 high. Objective magnification, 40×. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30134604), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - Validation of modulations in autophagy-related markers after SIRT6 knockdown in melanoma cells(A) RT-qPCR analysis was performed to validate the mRNA levels in key altered genes after SIRT6 knockdown A375 cells. Results are expressed as SIRT6 knockdown (shSIRT6) mRNA levels compared to nonsense control (shNS). Data are mean ±SE of three biological replicates, with statistical significance denoted as *P≤ 0.05. (B) Western blot analyses of various autophagy markers including LC3 I & II, BECN1, SQSTM1, ATG3, ATG7, ATG10 & GAA using cell lysates of shNS & shSIRT6 in A375 melanoma cells. beta-actin was used as loading control. The figure shows representative western blot images from 2-3 experiments. Image collected & cropped by CiteAb from the following publication (https://www.genesandcancer.com/lookup/doi/10.18632/genesandcancer.153), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - Fucoxanthin activated autophagy after TBI.(A) Representative images of immunofluorescence for LC3 surrounding the injured cortex. LC3 punctate dots were observed in the cytoplasm by immunofluorescent staining of LC3 (red). Neuron cells & nuclei are labeled with NeuN (green) & DAPI (blue), respectively. Magnification: 40 x. Scale bar: 50 mm. (B) Mice brain tissues were collected 1 day after TBI in different groups, & the expression of LC3, Beclin-1 & p62 was measured by western blot. Fucoxanthin treatment significantly increased the level of LC3-II & Beclin-1 while decreasing the level of p62 after TBI. (C) 3-MA (400 nM) was injected i.c.v. 30 min before TBI. Mice were then subjected to TBI & treatment of fucoxanthin 30 min after TBI. Pretreatment with 3-MA significantly attenuated fucoxanthin-induced activation of autophagy & suppression of apoptosis & oxidative stress in the ipsilateral cortex. Data are presented as mean ± SEM, n = 6 per group; **p < 0.01, ***p < 0.001 versus sham group; #p < 0.05, ##p < 0.01 versus TBI + vehicle group; &&p < 0.01, &&&p < 0.001 versus TBI + fucoxanthin group. beta-actin was used as a loading control. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28429775), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunocytochemistry/ Immunofluorescence: LC3B Antibody - BSA Free [NB600-1384] -
Immunocytochemistry/ Immunofluorescence: LC3B Antibody - BSA Free [NB600-1384] - Fucoxanthin activated autophagy after TBI.(A) Representative images of immunofluorescence for LC3 surrounding the injured cortex. LC3 punctate dots were observed in the cytoplasm by immunofluorescent staining of LC3 (red). Neuron cells & nuclei are labeled with NeuN (green) & DAPI (blue), respectively. Magnification: 40 x. Scale bar: 50 mm. (B) Mice brain tissues were collected 1 day after TBI in different groups, & the expression of LC3, Beclin-1 & p62 was measured by western blot. Fucoxanthin treatment significantly increased the level of LC3-II & Beclin-1 while decreasing the level of p62 after TBI. (C) 3-MA (400 nM) was injected i.c.v. 30 min before TBI. Mice were then subjected to TBI & treatment of fucoxanthin 30 min after TBI. Pretreatment with 3-MA significantly attenuated fucoxanthin-induced activation of autophagy & suppression of apoptosis & oxidative stress in the ipsilateral cortex. Data are presented as mean ± SEM, n = 6 per group; **p < 0.01, ***p < 0.001 versus sham group; #p < 0.05, ##p < 0.01 versus TBI + vehicle group; &&p < 0.01, &&&p < 0.001 versus TBI + fucoxanthin group. beta-actin was used as a loading control. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28429775), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - Striatal autophagic flux decreases from P10 to P28. (A) Schematic of coronal brain section showing dissection boundaries for ex vivo experiments. (B) Quantification of LC3B-ii relative to actin for every vehicle-only slice showed in Figures 4, 5. Unpaired, two-tailed t-test, t52 = 5.824, ****p < 0.0001. (C) Representative Western blot images of actin, DARPP32 & LC3B in slices obtained from P10 or P28 mice, incubated with BafA1 (100 nM, 3 h) or vehicle (Veh; DMSO, 0.1%). (D) LC3B-ii relative to actin, normalized to vehicle condition at each age. P10: unpaired, two-tailed t-test, t25 = 5.113, ****p < 0.0001; P28: unpaired, two-tailed t-test, t10 = 0.6228, p = 0.5473. P10: Veh: n = 16 slices, BafA1 n = 11 slices from 4 to 6 mice. P28: Veh: n = 6 slices, BafA1 n = 6 slices from three mice. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32296308), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - The activated HeyL-aromatase axis promotes autophagic survival of PCSCs. (A) Gene signatures associated with autophagy-associated signaling pathways identified by GSEA in HeyLHigh samples vs. HeyLLow samples from the TCGA-PRAD dataset & in HeyL knockdown cells vs. ctrl cells. (B, C) Western blot analysis of Beclin1, LC3, & CD44 in LNCaP (B) & PC3 (C) cells after the indicated treatments. (D) Flow cytometry analysis of the CD44+/CD24− subpopulation in PC3 cells treated as indicated. (E, F) mRNA (E) & protein (F) levels of stemness-associated genes & LC3 in PC3 cells after the indicated treatment (one-way ANOVA). (G, H) mRNA & protein levels of stemness-associated genes & autophagy-related genes in PC3 cells treated with ICI182780 (G) & siER alpha (H) (t-test). (I, J) Cleaved caspase3 levels in LNCaP & PC3 cells after the indicated treatments (one-way ANOVA). (K, L) Cleaved caspase3 levels in PC3 cells treated with ICI182780 (K) or transfected with siER alpha (L) (t-test). Bic, bicalutamide; CQ, chloroquine. The data are presented as the mean ± SD values (n=3). *p < 0.05 vs. ctrl. #p < 0.05 vs. OE-HeyL, i_HeyL or OE-CYP19A1. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/35096586), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - Treatment with gemcitabine (GCB) induces autophagy in osteosarcoma cellsA. Following 48 hours of treatment with 1μM GCB, LM7, CCH-OS-D, & K7M3 cells were stained with the lysosomotropic agent acridine orange (1 μg/ml) for 15 minutes & then visualized under a confocal microscope. Treatment with GCB led to the formation of acidic vesicular organelles (AVOs –red/orange), as shown in the representative images. B. Flow cytometry was used to measure & quantify AVO formation in untreated (left) & treated (right) osteosarcoma cells. C. Cells were treated with 1μM GCB for 48 hours, & after fixation, cells were stained with anti-LC3 antibody & analyzed by fluorescence confocal microscopy. GCB increases the number of green punctae in the treated group as compared to the untreated. D. Expression of BECN, LC3, & p62 was analyzed using Western blot analysis. Cells were treated with GCB for 48 hours, lysed & extracted protein was analyzed using specific antibodies. beta-actin was used as a loading control. Autophagy induction is demonstrated by an increase in the conversion of LC3I to LC3II, BECN expression & a decrease in p62 expression in the treated group as compared with the untreated. E. Treatment of OS cells with GCB (1μM, 48 hours), led to an increase in the formation of autophagic vacuoles (shown with red arrows) measured by transmission electron microscopy. Image collected & cropped by CiteAb from the following publication (https://www.oncotarget.com/lookup/doi/10.18632/oncotarget.20308), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - RAR alpha but not RAR beta & RAR gamma agonists induce autophagic flux in SKBR3 cells. (a) SKBR3 cells were treated with 1μM of RAR alpha, RAR beta or RAR gamma agonists for 2 days in the presence & absence of BafA during the last 2 h, before subjection to immunofluorescence for LC3B. (b) Quantification of LC3B puncta from the experiment described in (a). Three independent experiments were quantified as described in Schläfli et al.41 (c) Autophagic flux was determined from the immunofluorescence analysis shown in (a). N=3, Student's t-test & **P<0.01. (d) Long-lived protein degradation assay of control or cells treated with 1 μM of RAR alpha, RAR beta, RAR gamma agonists in the presence or absence of 200 nM BafA. Radioactivity was determined by liquid scintillation counting of four independent experiments. Data are shown as BafA-sensitive proteolysis. (e) Western blot analysis for VE-cadherin, beta-catenin & LC3B of SKBR3 cells treated as in (a). (f) LC3B-II levels on western blot were normalized to GAPDH & quantified from at least five independent experiments using the ImageJ software (NIH, Bethesda, MD, USA). LC3B-II levels of control treated cells were arbitrarily set to 100%. Mann–Whitney U-test: *P<0.05, **P<0.01 & ****P<0.0001 Image collected & cropped by CiteAb from the following publication (https://www.nature.com/articles/cddis2015236), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - ROS/AMPK/mTOR pathway was required for HMGB1-mediated autophagy regulating NIS expression (a) FTC-133/TPC-1 cells transfected with HMGB1 shRNA & control shRNA transfection were starved by HBSS for 3 h & then treated with rapamycin (1 μM) for 12 h. ROS production was assessed by measuring the fluorescent intensity of DCF on a fluorescent plate reader. Incremental production of ROS was expressed as a percentage of control. Rap, rapamycin. (n = 3, *P < 0.01, **P > 0.01); (b) FTC-133/TPC-1 cells transfected with HMGB1 shRNA & control shRNA were starved by HBSS for 3 h & then treated with rapamycin (1 μM) for 12 h. Flow cytometry was performed for measuring the ROS level by a DCFH-DA probe in indicated cells. Rap, rapamycin. (n = 3, *P < 0.01, **P > 0.01); (c) FTC-133/TPC-1 cells were transfected with HMGB1 shRNA & control shRNA & then starved by HBSS for 3 h. ATP levels were detected by ATP Assay Kit (n = 3, *P > 0.01); (d) FTC-133/TPC-1 cells were pretreated with NAC (2 mM) & then starved by HBSS for 3 h. LC3-I/II, NIS, p-AMPK, AMPK, p-mTOR, mTOR, p-p70S6K & p70S6K levels were assayed by Western blot; (e) FTC-133/TPC-1 cells transfected with HMGB1 shRNA & control shRNA were starved by HBSS for 3 h & then treated with rapamycin (1 μM) for 12 h. LC3-I/II, NIS, p-mTOR & mTOR levels were assayed by Western blot. Rap, rapamycin Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31331356), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - Serum starvation induces autophagy & apoptosis in endothelial cells.A Evaluation by Hoechst 33342 & propidium iodide (HO/PI) staining of apoptosis & necrosis in HUVECs exposed to normal medium (N) or serum-starved (SS) for 4 h. P values obtained by unpaired t test. n = 10 for each condition. Scale bar = 25 μm. B Quantification of caspase-3 activity in HUVECs exposed to N or SS HUVECs for 1, 2, 3, & 4 h. P values obtained by one-way ANOVA. n = 4 for each condition. C Immunoblot & densitometric analysis of PARP, LC3, & SQSTM1/p62 in HUVECs exposed to N or SS for 1, 2, or 4 h. beta-actin (ACTB) was used as a loading control. P values obtained by unpaired t test (*P < 0.05; **P < 0.01; ***P < 0.001). n = 3 for each condition. All values are expressed as the mean ± SEM. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/35149669), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - 4-phenylbutyric acid (4-PBA) alleviated zearalenone (ZEN)-induced ER stress, autophagy, & downregulation of CYP3A4 & UGT1A. Before treatment with ZEN (20 & 40 μg/mL), cells were pretreated with 1 mM 4-PBA for 6 h. mRNA & protein expression were determined by quantitative real-time PCR (qRT-PCR) & Western blot analysis, respectively. (A) Cells were treated with ZEN for 24 h after pretreatment with 4-PBA & cell viability was measured by the trypan blue dye exclusion test. (B) Phosphorylation of eIF2 alpha was evaluated in cells treated with ZEN for 2 h. The protein expression of (C) LC3-II/LC3-I & (D) CYP3A4 was determined in cells treated with ZEN for 24 h. (E) UGT1A mRNA levels were determined in cells treated with ZEN for 16 h. Data represent mean ± SEM of three independent experiments. * indicates significant difference vs. the control (* p < 0.05, ** p < 0.01, & *** p < 0.001). # indicates significant difference between groups (p < 0.05). Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31861425), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - Bmi‐1‐RING1B & autophagy are negatively related to SA‐PCH. (A) Kyoto Encyclopedia of Genes & Genomes (KEGG) analysis was conducted using differentially expressed genes (DEGs) in young (14‐week‐old) & old (18‐month‐old) mouse hearts. (B) Gene set enrichment analysis (GSEA) on autophagy signal pathway & ubiquitin‐mediated proteolysis. (C) Genes Correlation analysis using 12‐ & 18‐ month mouse hearts RNAseq count data. (D) Gene Ontology (GO) analysis & GSEA were processed using genes that were highly related to Bmi‐1. (E) Western blots of cardiac extracts of WT young (8‐week‐old) or aging (20‐month‐old) mice showing Bmi‐1, RING1B, LC3BII, Ang II, GATA4, ANP, BNP, NF‐ kappaB‐p65 & p‐p65 (Ser536), I kappaB‐ alpha & p‐I kappaB‐ alpha (Ser32); beta‐actin was the loading control. (F) Protein levels relative to beta‐actin were assessed by densitometric analysis. Six mice per group were used for experiments. Values are mean ± SEM of six determinations per group, **p < .01, ***p < .001 compared with the WT young mice, unpaired t‐test for bar graphs Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/35390228), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - The activated HeyL-aromatase axis promotes autophagic survival of PCSCs. (A) Gene signatures associated with autophagy-associated signaling pathways identified by GSEA in HeyLHigh samples vs. HeyLLow samples from the TCGA-PRAD dataset & in HeyL knockdown cells vs. ctrl cells. (B, C) Western blot analysis of Beclin1, LC3, & CD44 in LNCaP (B) & PC3 (C) cells after the indicated treatments. (D) Flow cytometry analysis of the CD44+/CD24− subpopulation in PC3 cells treated as indicated. (E, F) mRNA (E) & protein (F) levels of stemness-associated genes & LC3 in PC3 cells after the indicated treatment (one-way ANOVA). (G, H) mRNA & protein levels of stemness-associated genes & autophagy-related genes in PC3 cells treated with ICI182780 (G) & siER alpha (H) (t-test). (I, J) Cleaved caspase3 levels in LNCaP & PC3 cells after the indicated treatments (one-way ANOVA). (K, L) Cleaved caspase3 levels in PC3 cells treated with ICI182780 (K) or transfected with siER alpha (L) (t-test). Bic, bicalutamide; CQ, chloroquine. The data are presented as the mean ± SD values (n=3). *p < 0.05 vs. ctrl. #p < 0.05 vs. OE-HeyL, i_HeyL or OE-CYP19A1. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/35096586), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - HMGB1-mediated autophagy regulated NIS expression & iodide uptake (a). FTC-133/TPC-1 cells were pretreated with 3-MA (10 mM) treatment for 1 h & then starved by HBSS for 3 h. LC3-I/II & NIS levels were assayed by Western blot; (b&c) FTC-133/TPC-1 cells were transfected with HMGB1 shRNA & control shRNA & then starved by HBSS for 3 h. LC3-I/II & NIS levels were assayed by Western blot. & NIS mRNA was assayed by qRT-PCR (n = 3, *P > 0.01, **P > 0.01); (d) Dynamic uptaking: FTC-133/TPC-1 cells were transfected with HMGB1 shRNA & control shRNA & starved by HBSS for 3 h. Indicated cells were cultured with 175 KBq 131I for 5, l0, 20, 30, 60, 90 & 120 min. The uptake of 131I in indicated cells was detected by a gamma counter; (e) Radionuclide uptaking: FTC-133/TPC-1 cells were transfected with HMGB1 shRNA & control shRNA in the presence or absence of 3-MA (10 mM) treatment for 1 h & then starved by HBSS for 3 h. After 1-h incubating with 131I, the uptake of 131I in indicated cells was detected by a gamma counter (n = 3, *P < 0.01, **P > 0.01); (f) FTC-133/TPC-1 cells transfected with HMGB1 shRNA & control shRNA were starved by HBSS for 3 h & then treated with rapamycin (1 μM) for 12 h. After 1-h incubating with 131I, the uptake of 131I in indicated cells was detected by a gamma counter. Rap, rapamycin. (n = 3, *P < 0.01, **P > 0.01) Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31331356), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - ROS was sufficient for inducing HMGB1 translocation & enhancing autophagy (a) FTC-133/TPC-1 cells were pretreated with the antioxidant (NAC, 2 mM) for 1 h & then starved by HBSS for 3 h. ROS production was assessed by measuring the fluorescent intensity of DCF on a fluorescent plate reader. Incremental production of ROS was expressed as a percentage of control (n = 3, *P < 0.01, **P < 0.01). UT, untreated group; (b) Antioxidant & SOD1 RNAi regulated starvation-induced autophagy as measured by LC3-II expression. FTC-133/TPC-1 cells were pretreated with NAC (2 mM) for 1 h or SOD1 RNAi for 48 h, & then starved by HBSS for 3 h. LC3-I/II level was assayed by Western blot. (c) Antioxidant & SOD1 RNAi regulated starvation-induced HMGB1 translocation. FTC-133/TPC-1 cells were pretreated with NAC (2 mM) for 1 h or SOD1 RNAi for 48 h, & then starved by HBSS for 3 h. & the expression of nuclear/cytosolic HMGB1 was assayed by Western blot. Fibrillarin was a nuclear fraction control & tubulin a cytoplasmic fraction control Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31331356), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - Autophagy was induced under conditions of LH & inhibited by CQ & 3-MA in HCC cells.(A–C). SMMC-7721 & HepG2 cells were directly incubated under normal or LH conditions for 8 h or incubated under LH conditions in the presence or absence of CQ or 3-MA for 8 h. (A). The cells were transfected with GFP-tagged LC3 & observed with fluorescence microscopy. Arrows show the punctate GFP-LC3 fluorescence in the cytoplasm. (B). The number of punctate GFP-LC3 in each SMMC-7721 or HepG2 cell was counted, & at least 100 cells were included for each group. The data represent the mean ± SD based on three independent experiments. **p < 0.01. (C). The whole-cell lysates were subjected to western blot analysis as described in the ‘Materials & methods'section. The experiments were repeated at least three times. Image collected & cropped by CiteAb from the following publication (https://www.nature.com/articles/srep05382), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - ROS/AMPK/mTOR pathway was required for HMGB1-mediated autophagy regulating NIS expression (a) FTC-133/TPC-1 cells transfected with HMGB1 shRNA & control shRNA transfection were starved by HBSS for 3 h & then treated with rapamycin (1 μM) for 12 h. ROS production was assessed by measuring the fluorescent intensity of DCF on a fluorescent plate reader. Incremental production of ROS was expressed as a percentage of control. Rap, rapamycin. (n = 3, *P < 0.01, **P > 0.01); (b) FTC-133/TPC-1 cells transfected with HMGB1 shRNA & control shRNA were starved by HBSS for 3 h & then treated with rapamycin (1 μM) for 12 h. Flow cytometry was performed for measuring the ROS level by a DCFH-DA probe in indicated cells. Rap, rapamycin. (n = 3, *P < 0.01, **P > 0.01); (c) FTC-133/TPC-1 cells were transfected with HMGB1 shRNA & control shRNA & then starved by HBSS for 3 h. ATP levels were detected by ATP Assay Kit (n = 3, *P > 0.01); (d) FTC-133/TPC-1 cells were pretreated with NAC (2 mM) & then starved by HBSS for 3 h. LC3-I/II, NIS, p-AMPK, AMPK, p-mTOR, mTOR, p-p70S6K & p70S6K levels were assayed by Western blot; (e) FTC-133/TPC-1 cells transfected with HMGB1 shRNA & control shRNA were starved by HBSS for 3 h & then treated with rapamycin (1 μM) for 12 h. LC3-I/II, NIS, p-mTOR & mTOR levels were assayed by Western blot. Rap, rapamycin Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31331356), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - Fucoxanthin activated autophagy after TBI.(A) Representative images of immunofluorescence for LC3 surrounding the injured cortex. LC3 punctate dots were observed in the cytoplasm by immunofluorescent staining of LC3 (red). Neuron cells & nuclei are labeled with NeuN (green) & DAPI (blue), respectively. Magnification: 40 x. Scale bar: 50 mm. (B) Mice brain tissues were collected 1 day after TBI in different groups, & the expression of LC3, Beclin-1 & p62 was measured by western blot. Fucoxanthin treatment significantly increased the level of LC3-II & Beclin-1 while decreasing the level of p62 after TBI. (C) 3-MA (400 nM) was injected i.c.v. 30 min before TBI. Mice were then subjected to TBI & treatment of fucoxanthin 30 min after TBI. Pretreatment with 3-MA significantly attenuated fucoxanthin-induced activation of autophagy & suppression of apoptosis & oxidative stress in the ipsilateral cortex. Data are presented as mean ± SEM, n = 6 per group; **p < 0.01, ***p < 0.001 versus sham group; #p < 0.05, ##p < 0.01 versus TBI + vehicle group; &&p < 0.01, &&&p < 0.001 versus TBI + fucoxanthin group. beta-actin was used as a loading control. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28429775), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - Combination treatment of valproic acid (VPA) & doxorubicin (DOX) synergistically augmented the autophagy of HepG2 cells. (A) Acridine orange (AO) staining was used to detect acidic vesicles in HepG2 cells at the indicated concentration of VPA & DOX monotherapies & combination treatment after incubation for 48 h. Images were taken using fluorescence inverted microscopy. Red color represents acidic vesicle & green color represents non-acidic vesicle. Scale bar represents 200 μm; (B) The viability of HepG2 cells was analyzed after 48-h incubation in the indicated experimental condition by using EZ-Cytox assay; (C) Percentages (%) of AO-positive cells were counted in different fields (containing at least 40 cells per field); (D) LC3 I & II protein levels were analyzed using Western blotting. Actin was used as the loading control. The intensity of LC3B-II bands was quantified by scanning densitometry program ImageJ & normalized to that of actin (right panel). Three independent experiments were performed & results reported as the mean ± standard deviation (SD). ** p < 0.01, *** p < 0.001 compared with the control group. Image collected & cropped by CiteAb from the following publication (http://www.mdpi.com/1422-0067/18/5/1048), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - HMGB1-mediated autophagy regulated NIS expression & iodide uptake (a). FTC-133/TPC-1 cells were pretreated with 3-MA (10 mM) treatment for 1 h & then starved by HBSS for 3 h. LC3-I/II & NIS levels were assayed by Western blot; (b&c) FTC-133/TPC-1 cells were transfected with HMGB1 shRNA & control shRNA & then starved by HBSS for 3 h. LC3-I/II & NIS levels were assayed by Western blot. & NIS mRNA was assayed by qRT-PCR (n = 3, *P > 0.01, **P > 0.01); (d) Dynamic uptaking: FTC-133/TPC-1 cells were transfected with HMGB1 shRNA & control shRNA & starved by HBSS for 3 h. Indicated cells were cultured with 175 KBq 131I for 5, l0, 20, 30, 60, 90 & 120 min. The uptake of 131I in indicated cells was detected by a gamma counter; (e) Radionuclide uptaking: FTC-133/TPC-1 cells were transfected with HMGB1 shRNA & control shRNA in the presence or absence of 3-MA (10 mM) treatment for 1 h & then starved by HBSS for 3 h. After 1-h incubating with 131I, the uptake of 131I in indicated cells was detected by a gamma counter (n = 3, *P < 0.01, **P > 0.01); (f) FTC-133/TPC-1 cells transfected with HMGB1 shRNA & control shRNA were starved by HBSS for 3 h & then treated with rapamycin (1 μM) for 12 h. After 1-h incubating with 131I, the uptake of 131I in indicated cells was detected by a gamma counter. Rap, rapamycin. (n = 3, *P < 0.01, **P > 0.01) Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31331356), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - Metformin treatment increases cisplatin effects; (A) MDA-MB-231 & Ca Ski cells were either left untreated or treated with metformin or cisplatin alone or metformin in combination with cisplatin for 15 days. At the end of the treatment, cells were processed to obtain whole cell lysates. MAP1LC3B, pPRKAA2Thr172, PRKAA2 & CASP3 (cleaved) expression was determined by Western blot. ACTB was used as loading control. Densitometric analysis of the gels was performed as described under Materials & Methods; (B) MDA-MB-231 & Ca Ski cells were either left untreated or treated with metformin or cisplatin alone or metformin in combination with cisplatin for 15 days. Ammonia level in the culture medium was measured as indicated in the Material & Methods section. # Significantly different from treatment with metformin alone. All experiments in this figure were repeated three times. * Significantly different from control untreated cells. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30646605), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - Autophagy & the proteasome regulate CagA stability. (A) Wild-type (WT) & autophagy-deficient (Atg5−/−) MEFs were infected with a CagA+ vacA− isogenic mutant strain (MOI 100) for 8 hours using a gentamycin protection assay. The autophagy marker LC3-II was used to confirm that Atg5−/− MEFs are autophagy-deficient. CagA protein levels were measured by Western blotting using beta-actin as loading control. Graph shows fold change of CagA normalized to beta-actin relative to WT MEFs (mean + SEM; n = 4). (B) AGS cells were infected with a CagA+ vacA− isogenic mutant strain (MOI 50) for 19 hours using a gentamycin protection assay. MG132 (5 μM) was added during the 14-hour low-dose gentamycin incubation. DMSO was used as a vehicle control. CagA protein levels were measured by Western blotting using beta-actin as loading control. Graph shows fold change of CagA normalized to beta-actin relative to vehicle control (mean + SEM; n = 6). (C) AGS cells were infected with a CagA+ vacA− isogenic mutant strain (MOI 50) for 24 hours using a gentamycin protection assay. Lactacystin (10 μM) was added during the 19-hour low-dose gentamycin incubation. Water was used as a vehicle control. CagA protein levels were measured by Western blotting using beta-actin as loading control. Graph shows fold change of CagA normalized to beta-actin relative to vehicle control (mean + SEM; n = 5). Relevant gel bands were cropped from the original blots. Dotted line indicates slicing of two regions together from the same blot. Statistical analysis was performed using Student’s t-test. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30631092), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - Vps34 activity is required for maintaining LC3B-ii levels at P10 & mTOR inhibition increases p-Beclin-1 & LC3B-ii levels at P28. (A) Representative Western blot images for actin, DARPP32 & LC3B-i & -ii in striatal slices obtained from P10 or P28 mice, treated with SAR405 (1 μM) or vehicle (Veh; DMSO, 0.1%). (B,C) Quantification of LC3B-ii relative to actin, normalized to vehicle condition at each age. (B) P10: unpaired, two-tailed t-test, t11 = 2.985, p = 0.0124; P28: unpaired, two-tailed t-test, t24 = 1.807, p = 0.0922. P10: Veh: n = 6 slices, SAR405 n = 7 slices from 3 to 4 mice/age. (C) P28: Veh: n = 9 slices, Baf n = 7 slices from 3 to 4 mice/age. *p < 0.05. (D) Representative Western blot images for actin, DARPP32, p-Beclin-1 Ser14 & LC3B-i & -ii in striatal slices from P28 mice, treated with Torin-1 (5 μM) or vehicle (Veh; DMSO, 0.1%). Quantification of (E) LC3B-ii & (F) p-Beclin S14 relative to actin, normalized to vehicle. Data analyzed with unpaired, two-tailed t-test; (E) LC3B-ii/actin, t24 = 2.853, p = 0.0088. Veh: n = 19 slices, Baf n = 7 slices from 5 to 7 mice. *p < 0.05. (F) p-Beclin S14/actin, t22 = 3.337, p = 0.0030. **p < 0.01, Veh: n = 18 slices, Torin-1 n = 6 slices from 3 to 5 mice. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32296308), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - After stimulation with the NOD1 ligand, macrophages increased their expression of autophagy proteins Atg9, LC3 & IRGM. Alveolar macrophages (AMs), monocyte-derived macrophages (MDMs) & monocytes (MNs) were incubated in the presence of 5 μg/ml of Tri-DAP for 24 h. Cells were pre-incubated with Rip2/p38 inhibitor SB203580 (SB) for 30 min prior to Tri-DAP stimulation to block NOD1-mediated responses, as indicated. Atg9 & LC3 proteins were measured in the cytosolic fractions by western blot analysis (A). The fold increases relative to the unstimulated cells were calculated after being normalized to tubulin, mean ± SE is depicted, n = 3 (B, C). The up-regulation of IRGM gene expression was assessed after specific ligand recognition by quantitative PCR using the Taqman system & delta deltaCT method for relative quantification. The fold changes in gene expression relative to the unstimulated cells of at least 6 subjects are depicted; bold lines indicate medians, *p < 0.05 (D). IRGM protein was measured in the cytosolic fractions of three subjects by western blot, & the fold increases relative to the unstimulated cells are indicated after being normalized to tubulin; mean ± SE is depicted, n = 3 (E, F). Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/25253572), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - Vps34 activity is required for maintaining LC3B-ii levels at P10 & mTOR inhibition increases p-Beclin-1 & LC3B-ii levels at P28. (A) Representative Western blot images for actin, DARPP32 & LC3B-i & -ii in striatal slices obtained from P10 or P28 mice, treated with SAR405 (1 μM) or vehicle (Veh; DMSO, 0.1%). (B,C) Quantification of LC3B-ii relative to actin, normalized to vehicle condition at each age. (B) P10: unpaired, two-tailed t-test, t11 = 2.985, p = 0.0124; P28: unpaired, two-tailed t-test, t24 = 1.807, p = 0.0922. P10: Veh: n = 6 slices, SAR405 n = 7 slices from 3 to 4 mice/age. (C) P28: Veh: n = 9 slices, Baf n = 7 slices from 3 to 4 mice/age. *p < 0.05. (D) Representative Western blot images for actin, DARPP32, p-Beclin-1 Ser14 & LC3B-i & -ii in striatal slices from P28 mice, treated with Torin-1 (5 μM) or vehicle (Veh; DMSO, 0.1%). Quantification of (E) LC3B-ii & (F) p-Beclin S14 relative to actin, normalized to vehicle. Data analyzed with unpaired, two-tailed t-test; (E) LC3B-ii/actin, t24 = 2.853, p = 0.0088. Veh: n = 19 slices, Baf n = 7 slices from 5 to 7 mice. *p < 0.05. (F) p-Beclin S14/actin, t22 = 3.337, p = 0.0030. **p < 0.01, Veh: n = 18 slices, Torin-1 n = 6 slices from 3 to 5 mice. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32296308), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunocytochemistry/ Immunofluorescence: LC3B Antibody - BSA Free [NB600-1384] -
Immunocytochemistry/ Immunofluorescence: LC3B Antibody - BSA Free [NB600-1384] - The effect of H19 siRNA on autophagy in OGD/R model. A) Immunofluorescence of LC3II in different conditions. B) Immunofluorescence of Beclin1 in different conditions. C) Immunofluorescence of P62 in different conditions. D) The change of lncRNA H19 expression level induced by H19 siRNA. E. Western blot of LC3II, LC3I, Beclin1, & P62 in different groups. F, G, & H) Statistical analysis of the expression of LC3II, Beclin1 & P62, respectively. N, normal group; OGD/R, OGD 8 hr & reperfusion 24 hr; OGD/R + H19 siRNA & OGD/R + N.C, transfecting the cells with H19 siRNA & normal control siRNA, respectively, before OGD/R treatment. Bar represents the mean value ± SD. *p<0.05 vs. normal group, #p<0.05 vs. OGD/R group, &p<0.05 vs. OGD/R + H19 siRNA group. White arrows indicate the co-localized positive cells. Image collected & cropped by CiteAb from the following publication (http://www.aginganddisease.org/EN/10.14336/AD.2016.0530), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - Inhibition of autophagy impacts on ATRA-induced epithelial differentiation of SKBR3 cells. (a) SKBR3 cells were treated for 4 days with 1 μM ATRA, increasing concentrations of chloroquine or a combination thereof as indicated before western blot analysis for VE-cadherin, beta-catenin, LC3B & GAPDH. (b) Quantification of beta-catenin & VE-cadherin western blots as one is shown in (a). Raw values were normalized to GAPDH & levels of ATRA-treated control transduced cells were arbitrary set to 100% as signals were not always detectable in vehicle-treated control cells. Bars & s.d. representative of five independent experiments. (c) SKBR3 control & ATG7-depleted cells were subjected to 1 μM ATRA for 4 days & expression levels of VE-cadherin, beta-catenin, ATG7 & GAPDH were determined by western blot. (d) Quantification of western blots for beta-catenin & VE-cadherin. Normalization was performed as described in (b) Image collected & cropped by CiteAb from the following publication (https://www.nature.com/articles/cddis2015236), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - Autophagy controls the cleavage of perlecan to LG3 fragments in apoptotic exosome-like vesicles (ApoExos).A Serum anti-LG3 IgG titers in allografted mice after 3 weeks of intravenous injections with vehicle (Ctrl) or ApoExos produced by serum-starved mECs treated with vehicle (ApoExo V), bafilomycin A1 (20 nM, ApoExo Baf), or ZVAD-FMK (50 μM, ApoExo ZVAD). P values were obtained by one-tailed unpaired t test. ns (nonsignificant). n = 7 mice for V & Baf injections, & n = 9 mice for V & ZVAD-FMK injections. B Representation of the abundance of perlecan peptides in ApoExos from serum-starved mECs treated with vehicle (V) or bafilomycin A1 (20 nM, Baf). Fold enrichment of perlecan peptide intensity in ApoExos from bafilomycin-treated cells compared to ApoExos from vehicle-treated cells. C Representative immunoblots & densitometric analysis of perlecan & LG3 fragments in ApoExos purified from HUVECs serum starved (SS) for 4 h in the presence of V or Baf. Ponceau red was used as a loading control. P values were obtained by the unpaired t test. n = 3 for each condition. *P < 0.05; **P < 0.01; ***P < 0.001. All values are expressed as the mean ± SEM. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/35149669), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - Induction of autophagic flux in OE19 upon Lapatinib treatment. (a) LC3B flux was assessed comparing control & BafilomycinA (BafA)-treated (200 nM, 2 h) OE19 upon Lapatinib treatment (120 nM, 24 h). LC3 band intensities were quantified using ImageJ software. Total protein was used as a loading control, & Phospho-Her2 for Lapatinib treatment (n = 3). (b) LC3B flux was calculated from data in (a) as follows: BafA+-BafA− values for each condition. (c) FACS analysis of mCherry-EGFP-LC3B-expressing OE19 cells upon induction or blockade of autophagy with indication of the chosen cut-off value for high respectively low autophagic flux. (d) Quantification of the FACS analysis showing % of cells with high autophagic flux (n = 3). Cells were treated as in (a). The error bars represent SD, statistical significance was determined by Mann–Whitney U test: * p ≤ 0.05, ** p ≤ 0.01. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30297650), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - Autophagy regulates the loading of LG3 on apoptotic exosome‐like vesicles (ApoExos).A Representative immunoblots & densitometric analysis of LC3, ATG7, phospho-AKT1, & total AKT1 from serum-starved (SS) HUVECs transfected with siCtrl or siATG7 or exposed to vehicle (V), wortmannin (100 nM; Wort), or bafilomycin A1 (20 nM, Baf). beta-actin (ACTB) was used as a loading control. n = 3 for each condition. B HO/PI staining of apoptosis in SS HUVECs transfected with siCtrl or siATG7 or exposed to V, Baf, or Wort. n = 3–5 for each condition. ns (nonsignificant). C Immunoblots & densitometric analysis of cleaved caspase-3 in SS HUVECs transfected with siCtrl or siATG7 or exposed to V, Baf, or Wort. beta-actin (ACTB) was used as a loading control. n = 3–5 for each condition. D Small particle flow cytometric quantifications of CMFDA+ AnnexinV+ ApoExos detected in the supernatant of HUVECs serum starved (SS) for 4 h & transfected with siCtrl or siATG7 or exposed to V, Baf, or Wort. n = 3 for each condition. E Representative immunoblots & densitometric analysis of LG3, 20S proteasome, LAMP2, & synthenin-1 in ApoExos purified from HUVECs serum starved (SS) for 4 h & transfected with siCtrl or siATG7 or exposed to V, Baf, or Wort. Ponceau red was used as a loading control. n = 3–5 for each condition. P values were obtained by the unpaired t test (*P < 0.05; **P < 0.01; ***P < 0.001). All values are expressed as the mean ± SEM. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/35149669), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunocytochemistry/ Immunofluorescence: LC3B Antibody - BSA Free [NB600-1384] -
Immunocytochemistry/ Immunofluorescence: LC3B Antibody - BSA Free [NB600-1384] - Treatment with gemcitabine (GCB) induces autophagy in osteosarcoma cellsA. Following 48 hours of treatment with 1μM GCB, LM7, CCH-OS-D, & K7M3 cells were stained with the lysosomotropic agent acridine orange (1 μg/ml) for 15 minutes & then visualized under a confocal microscope. Treatment with GCB led to the formation of acidic vesicular organelles (AVOs –red/orange), as shown in the representative images. B. Flow cytometry was used to measure & quantify AVO formation in untreated (left) & treated (right) osteosarcoma cells. C. Cells were treated with 1μM GCB for 48 hours, & after fixation, cells were stained with anti-LC3 antibody & analyzed by fluorescence confocal microscopy. GCB increases the number of green punctae in the treated group as compared to the untreated. D. Expression of BECN, LC3, & p62 was analyzed using Western blot analysis. Cells were treated with GCB for 48 hours, lysed & extracted protein was analyzed using specific antibodies. beta-actin was used as a loading control. Autophagy induction is demonstrated by an increase in the conversion of LC3I to LC3II, BECN expression & a decrease in p62 expression in the treated group as compared with the untreated. E. Treatment of OS cells with GCB (1μM, 48 hours), led to an increase in the formation of autophagic vacuoles (shown with red arrows) measured by transmission electron microscopy. Image collected & cropped by CiteAb from the following publication (https://www.oncotarget.com/lookup/doi/10.18632/oncotarget.20308), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - The activated HeyL-aromatase axis promotes autophagic survival of PCSCs. (A) Gene signatures associated with autophagy-associated signaling pathways identified by GSEA in HeyLHigh samples vs. HeyLLow samples from the TCGA-PRAD dataset & in HeyL knockdown cells vs. ctrl cells. (B, C) Western blot analysis of Beclin1, LC3, & CD44 in LNCaP (B) & PC3 (C) cells after the indicated treatments. (D) Flow cytometry analysis of the CD44+/CD24− subpopulation in PC3 cells treated as indicated. (E, F) mRNA (E) & protein (F) levels of stemness-associated genes & LC3 in PC3 cells after the indicated treatment (one-way ANOVA). (G, H) mRNA & protein levels of stemness-associated genes & autophagy-related genes in PC3 cells treated with ICI182780 (G) & siER alpha (H) (t-test). (I, J) Cleaved caspase3 levels in LNCaP & PC3 cells after the indicated treatments (one-way ANOVA). (K, L) Cleaved caspase3 levels in PC3 cells treated with ICI182780 (K) or transfected with siER alpha (L) (t-test). Bic, bicalutamide; CQ, chloroquine. The data are presented as the mean ± SD values (n=3). *p < 0.05 vs. ctrl. #p < 0.05 vs. OE-HeyL, i_HeyL or OE-CYP19A1. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/35096586), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - Activation of autophagy by MG132 is associated with AGR2 decline. a The expression change of AGR2 in A549 cells was estimated by western blotting analysis. A549 cells were incubated with CHX (2 μg/ml) for 2 h prior to MG132 treatment. GAPDH served as a loading control. b AGR2 ubiquitination was detected by immunoprecipitation with anti-AGR2 antibody & immunoblotting with anti-Ub antibody. Similar results were obtained with three independent experiments. c Immunofluorescence microscopy for analysis of punctate pattern of LC3 localization in A549 cells transiently transfected with the GFP-LC3-vector for 24 h & treated with MG132 for additional 12 or 24 h, & LC3 punctate (right panel) was calculated in A549 cells. **p < 0.01 compared with the control. Scale bar = 20 μm. d The levels of AGR2 & LC3B were measured by western blotting with MG132 treatment at indicated time. e Analysis of LC3B & AGR2 accumulation in A549 cells by fluorescent staining. Scale bar = 10 μm Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30647455), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - VPA reduces autophagy at P28. (A) Schematic of VPA administration. (B) Representative Western blot images for LC3B, p62, p-rpS6 S240/244, DARPP32, & actin. (B–G) Quantification of (B–D) p-rpS6 S240/244 relative to actin, (B,E,F) LC3B-ii relative to actin (B,G,H) p62 & (B,I,J) DARPP32 normalized to VPA untreated (veh, vehicle) mice at P10 & P28, respectively. Data analyzed with unpaired, two-tailed t-test; (C) p-rpS6 S240/244/actin at P10, ns; (D) p-rpS6 S240/244/actin at P28, t11 = 2.882, p = 0.0149 (E) LC3B-ii/actin at P10, ns; (F) LC3B-ii/actin at P28, t10 = 2.231, p = 0.047; (G) p62 at P10, ns; (H) p62 at P28, t10 = 3.752, p = 0.0038; (I) DARPP32 at P10, ns, & (J) DARPP32 at P10, ns, *p < 0.05, **p < 0.01, ns not significant, n = 4–7 mice/treatment. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32296308), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - The activated HeyL-aromatase axis promotes autophagic survival of PCSCs. (A) Gene signatures associated with autophagy-associated signaling pathways identified by GSEA in HeyLHigh samples vs. HeyLLow samples from the TCGA-PRAD dataset & in HeyL knockdown cells vs. ctrl cells. (B, C) Western blot analysis of Beclin1, LC3, & CD44 in LNCaP (B) & PC3 (C) cells after the indicated treatments. (D) Flow cytometry analysis of the CD44+/CD24− subpopulation in PC3 cells treated as indicated. (E, F) mRNA (E) & protein (F) levels of stemness-associated genes & LC3 in PC3 cells after the indicated treatment (one-way ANOVA). (G, H) mRNA & protein levels of stemness-associated genes & autophagy-related genes in PC3 cells treated with ICI182780 (G) & siER alpha (H) (t-test). (I, J) Cleaved caspase3 levels in LNCaP & PC3 cells after the indicated treatments (one-way ANOVA). (K, L) Cleaved caspase3 levels in PC3 cells treated with ICI182780 (K) or transfected with siER alpha (L) (t-test). Bic, bicalutamide; CQ, chloroquine. The data are presented as the mean ± SD values (n=3). *p < 0.05 vs. ctrl. #p < 0.05 vs. OE-HeyL, i_HeyL or OE-CYP19A1. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/35096586), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunocytochemistry/ Immunofluorescence: LC3B Antibody - BSA Free [NB600-1384] -
Immunocytochemistry/ Immunofluorescence: LC3B Antibody - BSA Free [NB600-1384] - In vitro alphaS seeds induce endo-lysosome-free & endo-lysosome-associated alphaS inclusions in H4/V1S-SV2 cells. (a) Gel analysis of in vitro prepared alphaS seeds. Recombinant alphaS solution subjected to 7 days of shaking was ultracentrifuged (110,000 × g, 20 min), & the resultant supernatant & pellet were resolved by SDS-PAGE & western blotting with anti-alpha S antibody (Synuclein-1, 610787, BD Biosciences). A second aliquot without shaking was included as a control. (b) Electron micrographs of alphaS seeds before & after sonication. Scale bar: 100 nm. Different alphaS polymorphs are shown in close-ups. (c) H4/V1S-SV2 cells treated with sonicated alphaS seeds for 2 days were subjected to immunocytochemistry with primary antibody against LAMP1 followed by DAPI counterstain to demonstrate the association between endo-lysosomes & seeded alphaS inclusions. The bottom two rows are magnified images of frames A & B in the second row. Empty triangles & solid arrowheads denote the endo-lysosome-free & endo-lysosome-associated alphaS inclusions, respectively. Scale bar: 10 µm (top two rows) & 5 µm (bottom two rows). (d) The treated cells were further subjected to immunocytochemical staining of LAMP1 (Alexa fluor 405) & LC3 (Alexa fluor 568) to demonstrate the association between autophagosomes, endo-lysosomes, & seeded alphaS inclusions. Scale bar: 5 µm (top row) & 2 µm (bottom row). Image collected & cropped by CiteAb from the following publication (https://www.nature.com/articles/s41598-017-08149-w), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - CA1 Tg mice undergo mitochondrial stress. a WB analysis of extracts from 6-mo Wt & Tg mice CA1 (Wt, n = 6; Tg, n = 7), using antibodies against SIRT3, SOD2 & acetylated SOD2. b WB analysis of extracts from 12-mo Wt & Tg mice hippocampi (Wt, n = 5; Tg, n = 5). Data are presented as mean ± SEM (**P < 0.01; ***P < 0.001). WB analysis of extracts from 6-mo Wt & Tg mice CA1 (Wt, n = 6; Tg, n = 5) (c), & from 12-mo Wt & Tg mice hippocampi (Wt, n = 5; Tg, n = 5) (d) for PINK1 & LC-3. Data are presented as mean ± SEM, *P < 0.05. WB analysis of extracts from 6-mo WT & Tg mice CA1 (WT, n = 5; Tg, n = 6) (e), & from 12-mo WT & Tg mice hippocampi (WT, n = 5; Tg, n = 5) (f) for TFAM. Data are presented as mean ± SEM (*P < 0.05), **P < 0.01). Actin was used as loading control Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32131898), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - Effects of exogenous HMGB1 on ATRA-induced differentiationA. Viability of NB4 cells that were treated with exogenous HMGB1 (10, 20 & 50 μg/ml) for 6-72 h was determined by the CCK-8 assay. Viability of control cells (DMSO) was set as 100%. (n=3, *P <0.05 versus control group). B. LDH released by NB4 cells that were treated with HMGB1 (10 μg/ml) for 6-48 h was detected by LDH assay kit & expressed as percentage of control (n=3, *P>0.05 versus untreated group). C. Morphological features of NB4 cells that were treated with ATRA (1 μM) or HMGB1 (1 μg/ml) was determined by Wright-Giemsa staining & visualized by light microscopy (100X magnification). D. Differentiation of NB4 cells treated with ATRA (1 μM) or HMGB1 (10 μg/ml) for 6-48 h was examined by NBT reduction & surface CD11b expression in comparison to the control (DMSO) (n=3, *P<0.05 versus ATRA group). E. Expression of PML-RAR alpha, P62 & LC3-I/II in NB4 cells treated with ATRA (1 μM) or HMGB1 (10 μg/ml) for 48 h was assayed by western blotting & co-immunoprecipitation in comparison to control (DMSO). Image collected & cropped by CiteAb from the following publication (https://www.oncotarget.com/lookup/doi/10.18632/oncotarget.15432), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - Zearalenone (ZEN) induced endoplasmic reticulum (ER) stress & autophagy in HepG2 cells. mRNA level & protein expression were determined by quantitative real-time polymerase chain reaction (qRT-PCR) & Western blot analysis, respectively. (A) The protein expression level of GRP78 was measured in cells treated with ZEN for 1 h. (B) The phosphorylation of eIF2 alpha was evaluated in cells treated with ZEN for 2 h. (C) CHOP mRNA levels, (D) Beclin1 mRNA levels, & (E) LC3-II mRNA levels were determined in cells treated with ZEN for 4 h. (F) The protein expression level of LC3-II/LC3-I was examined in cells treated with ZEN for 24 h. Data represent mean ± SEM of three independent experiments. * indicates significant difference vs. the control (** p < 0.01, & *** p < 0.001). Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31861425), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - ATRA induces autophagy in SKBR3 cells. (a) SKBR3 & MDA-MB-453 breast cancer cells were treated either with 0.1 or 1 μM ATRA for 2 & 4 days, respectively. beta-Catenin & LC3B levels were measured by western blotting. GAPDH was used as a loading control. (b) LC3B-II quantification from at least three independent experiments. LC3B-II expression was normalized to GAPDH & to vehicle-treated SKBR3 cells. (c) beta-Catenin staining of SKBR3 & MDA-MB453 cells. Cells were control or ATRA (1 μM) treated for 2 days. beta-Catenin FITC (fluorescein isothiocyanate) & nuclear DAPI (4',6-diamidino-2-phenylindole) staining as analyzed by confocal microscopy are shown. (d) Quantification of endogenous LC3B puncta. LC3B in SKBR3 & MDA-MB453 cells treated with 1 μM ATRA for 2 days. (e) LC3B-II western blotting & quantification of the autophagic activation upon treatment with 1 μM ATRA for 2 days in the presence or absence of BafA for 2 h at 200 nM. LC3B-II levels were normalized to GAPDH & to vehicle-treated SKBR3 cells. Standard deviations for five independent experiments are shown. (f) Long-lived protein degradation assay of SKBR3 & MDA-MB453 cells treated as in (e). Radioactivity was determined by liquid scintillation counting of at least three independent experiments. Absolute proteolysis is shown. (g) Long-lived protein degradation assay of SKBR3 treated as in (e) & including treatment with the macroautophagy-specific inhibitor 3-MA (10 mM). Analysis as in (f). Mann–Whitney U-test: *P<0.05, **P<0.01, ***P<0.001 & ****P<0.0001 Image collected & cropped by CiteAb from the following publication (https://www.nature.com/articles/cddis2015236), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - CA1 Tg mice undergo mitochondrial stress. a WB analysis of extracts from 6-mo Wt & Tg mice CA1 (Wt, n = 6; Tg, n = 7), using antibodies against SIRT3, SOD2 & acetylated SOD2. b WB analysis of extracts from 12-mo Wt & Tg mice hippocampi (Wt, n = 5; Tg, n = 5). Data are presented as mean ± SEM (**P < 0.01; ***P < 0.001). WB analysis of extracts from 6-mo Wt & Tg mice CA1 (Wt, n = 6; Tg, n = 5) (c), & from 12-mo Wt & Tg mice hippocampi (Wt, n = 5; Tg, n = 5) (d) for PINK1 & LC-3. Data are presented as mean ± SEM, *P < 0.05. WB analysis of extracts from 6-mo WT & Tg mice CA1 (WT, n = 5; Tg, n = 6) (e), & from 12-mo WT & Tg mice hippocampi (WT, n = 5; Tg, n = 5) (f) for TFAM. Data are presented as mean ± SEM (*P < 0.05), **P < 0.01). Actin was used as loading control Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32131898), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - ATRA induces autophagy in SKBR3 cells. (a) SKBR3 & MDA-MB-453 breast cancer cells were treated either with 0.1 or 1 μM ATRA for 2 & 4 days, respectively. beta-Catenin & LC3B levels were measured by western blotting. GAPDH was used as a loading control. (b) LC3B-II quantification from at least three independent experiments. LC3B-II expression was normalized to GAPDH & to vehicle-treated SKBR3 cells. (c) beta-Catenin staining of SKBR3 & MDA-MB453 cells. Cells were control or ATRA (1 μM) treated for 2 days. beta-Catenin FITC (fluorescein isothiocyanate) & nuclear DAPI (4',6-diamidino-2-phenylindole) staining as analyzed by confocal microscopy are shown. (d) Quantification of endogenous LC3B puncta. LC3B in SKBR3 & MDA-MB453 cells treated with 1 μM ATRA for 2 days. (e) LC3B-II western blotting & quantification of the autophagic activation upon treatment with 1 μM ATRA for 2 days in the presence or absence of BafA for 2 h at 200 nM. LC3B-II levels were normalized to GAPDH & to vehicle-treated SKBR3 cells. Standard deviations for five independent experiments are shown. (f) Long-lived protein degradation assay of SKBR3 & MDA-MB453 cells treated as in (e). Radioactivity was determined by liquid scintillation counting of at least three independent experiments. Absolute proteolysis is shown. (g) Long-lived protein degradation assay of SKBR3 treated as in (e) & including treatment with the macroautophagy-specific inhibitor 3-MA (10 mM). Analysis as in (f). Mann–Whitney U-test: *P<0.05, **P<0.01, ***P<0.001 & ****P<0.0001 Image collected & cropped by CiteAb from the following publication (https://www.nature.com/articles/cddis2015236), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - Bmi‐1 deficiency aggravates cardiac hypertrophy & decreases autophagic flux. (A) Representative micrographs of paraffin‐embedded heart ventricular wall sections of 6‐week‐old Bmi‐1–/– mice & WT mice stained for haematoxylin‐eosin (H&E), wheat germ agglutinin (WGA) & Masson's trichrome (Masson) staining. (B) Heart weight relative to body weight (HW/BW) & heart weight relative to tibia length (HW/TL). (C) The wall thickness of interventricular septum. (D) Myocyte cross‐sectional area from Bmi‐1–/– mice relative to WT mice. (E) Collagenous fibre area from Bmi‐1–/– mice relative to WT mice was counted with anterior wall staining for Masson. (F) Anp, Bnp, beta‐MHC, c‐TnI & GATA4 mRNA levels in hearts by real‐time RT‐PCR, calculated as ratio to beta‐actin mRNA & expressed relative to WT. (G) Western blots of cardiac tissue extracts & mouse embryonic cardiomyocytes (MECs) showing ANP, BNP, GATA4, LC3B & p16; beta‐actin was the loading control. (H) Protein levels relative to beta‐actin were assessed by densitometric analysis. (I) Representative micrographs of paraffin‐embedded heart sections immunohistochemical staining for ANP, BNP & GATA4. (J) The percentage of cells positive for ANP, BNP, GATA4 or positive area relative to total cells or area. (K) Representative micrographs of fluorescence with autophagy lentivirus transfection, with DAPI for nuclei. (L) Percentage of positive GFP dots or mRFP dots relative to total cells. (M) The number of autophagosomes & autolysosomes per cell. Six mice per group were used for experiments. Cell experiments were performed with three biological repetitions per group. Values are mean ± SEM from six determinations per group, *p < .05, **p < .01, ***p < .001 compared with WT group, unpaired Student's t‐test Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/35390228), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - 8-Cl-Ado-induces autophagy. Immunoblot analysis of (A) phospho-ULK (Ser555) levels & (B) LC3B-I lipidation to form LC3B-II in MCF-7 & BT-474 cells treated with 10 μM of 8-Cl-Ado for the indicated times. (C) Fluorescent microscopy of MCF-7 & BT-474 cells transiently transfected with GFP-LC3B & treated with 10 μM of 8-Cl-Ado for 12-hours to assess aggregation of LC3B. (D) Immunoblot analysis of p62 levels in MCF-7 & BT-474 cells treated with 10 μM of 8-Cl-Ado for the indicated times. The normalized ratio of p62 to GAPDH relative to the untreated control is indicated beneath each lane. (E) Fluorescent microscopic imaging of autolysosomes. MCF-7 cells were untreated or treated for 2-days with 8-Cl-Ado or rapamycin. Cells were stained with MDC, blue, to visualize AVO & Syto 61 (DNA), red, for nuclei counterstaining. (F) Representative flow cytometery histograms of AVO stained with acridine orange in MCF-7 cells untreated or treated for 3 days with 50 nM rapamycin or 10 μM 8-Cl-Ado. Baf was added before 30 min prior to staining to neutralize AVO staining. (G) Quantification triplicate experiments of MCF-7 & BT-474 cells treated & analyzed as in E. Image collected & cropped by CiteAb from the following publication (https://jhoonline.biomedcentral.com/articles/10.1186/1756-8722-7-23), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3B Antibody - BSA Free [NB600-1384] -
Western Blot: LC3B Antibody - BSA Free [NB600-1384] - PB induces autophagy but blocks autophagosome trafficking & autophagosome-lysosome fusion in Hep3B cells.(A) Hep3B cells, HepG2 cells, & primary mouse hepatocytes treated w/ increasing concentrations of PB (6–2000 μg/mL) for 24 h; viable cells quantified using the MTT assay. Data are presented as mean ± SD from at least 3 independent experiments. Bars = SD. *P < 0.05, **P < 0.01, ***P < 0.001, control versus PB-treated cells. (B) Hep3B cells treated for 6, 24 & 48 h w/ indicated concentrations of PB. Levels of protein expression analysed by WB using antibodies against LC3B, p62/SQSTM1, ATG12, BECN1 & Actin. Images cropped from different blots run under the same experimental conditions. The original blots attached as Supplementary Figure 3A. (C) Hep3B cells transfected w/ GFP-LC3B treated w/ 240 μg/mL PB for 24 h & then observed under a confocal microscope. (D) Hep3B cells treated for 24 h w/ indicated concentrations of PB. Levels of protein expression analysed by WB using antibodies against phosphorylated & total mTOR, 4EBP1, & p70S6K1 as well as actin. Images cropped from different blots run under the same experimental conditions. The original blots attached as Supplementary Figure 3B. (E) Hep3B cells serum-starved overnight, & incubated w/ or w/out PB (240 μg/mL) for 6 h before stimulating w/ 20 ng/mL EGF for 20 min. EGFR protein levels analysed by confocal microscope. (F) Hep3B cells treated PB for 12, 24 or 48 h w/ indicated concentrations. Levels of protein expression analysed by WB using antibodies against EGFR & Actin. Images cropped from different blots run under the same experimental conditions in each panel. The original blots attached as Supplementary Figure 3C. Image collected & cropped by CiteAb from the following publication (https://www.nature.com/articles/srep41437), licensed under a CC-BY license. Not internally tested by Novus Biologicals.

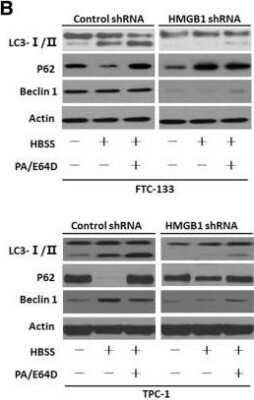

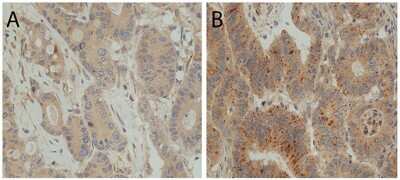
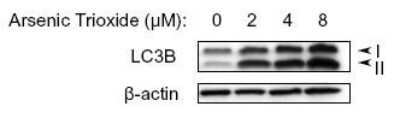
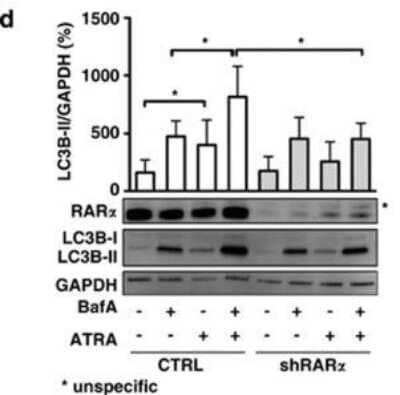
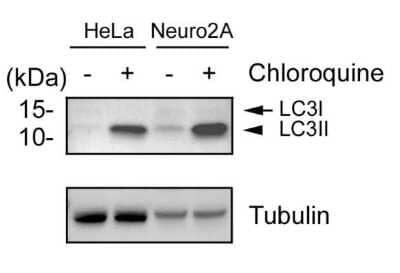

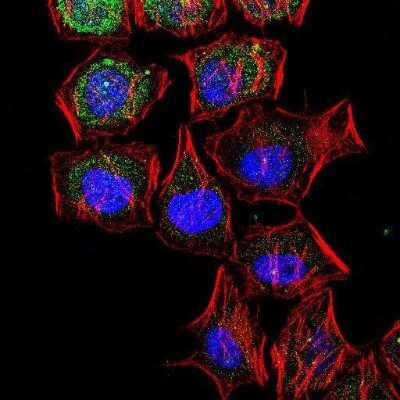
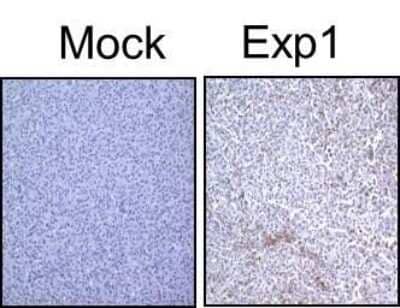
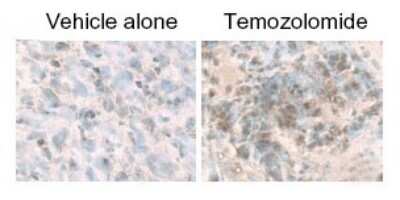
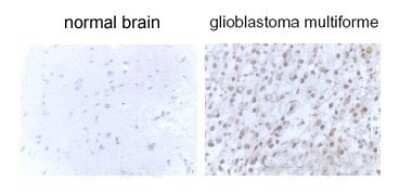

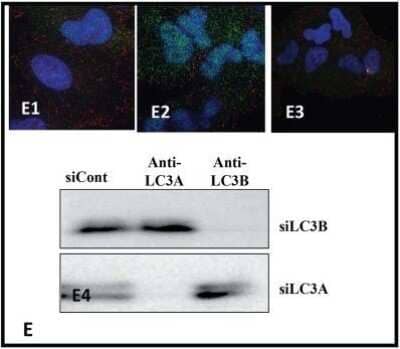
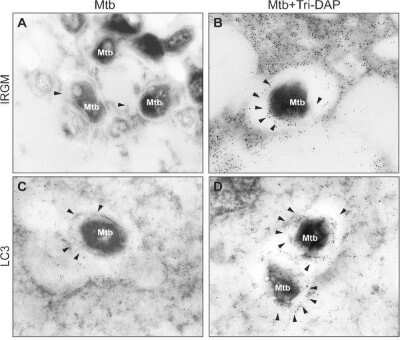
![Immunohistochemistry: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-271220231253359.jpg)
![Simple Western: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-30820241044551.jpg)
![Immunocytochemistry/ Immunofluorescence: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-210202423454831.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-210202423452429.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-210202423454878.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-210202423454836.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-210202423454816.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-210202423454824.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-210202423454888.jpg)
![Immunocytochemistry/ Immunofluorescence: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202415384116.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202415304227.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202415293333.jpg)
![Immunohistochemistry: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202415284541.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-31020241535679.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-31020241615466.jpg)
![Immunocytochemistry/ Immunofluorescence: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416175221.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-31020241616079.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416163736.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416175242.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-31020241616026.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-31020241617143.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-31020241616069.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416182621.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416163778.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416154615.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416161815.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416165628.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416163729.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416182641.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-31020241615464.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-31020241616039.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-31020241616376.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-31020241618938.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416173533.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416171441.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-31020241616061.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-31020241617524.jpg)
![Immunocytochemistry/ Immunofluorescence: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416163739.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-31020241616037.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-3102024161603.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416175251.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416171498.jpg)
![Immunocytochemistry/ Immunofluorescence: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416163748.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416163799.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416163789.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416171449.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416182610.jpg)
![Immunocytochemistry/ Immunofluorescence: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-31020241616186.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416175256.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416171499.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416154612.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-31020241616052.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-31020241616181.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-310202416182623.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-31020241618262.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-31020241617525.jpg)
![Western Blot: LC3B Antibody - BSA Free [NB600-1384] - LC3B Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb600-1384_rabbit-polyclonal-lc3b-antibody-31020241618948.jpg)