MDR1/ABCB1 Antibody (MRK16) - Azide and BSA Free
Novus Biologicals, part of Bio-Techne | Catalog # NBP2-52701
Recombinant Monoclonal Antibody

Conjugate
Catalog #
Key Product Details
Species Reactivity
Human
Applications
CyTOF-ready, Flow Cytometry, Immunocytochemistry/ Immunofluorescence, Immunoprecipitation
Label
Unconjugated
Antibody Source
Recombinant Monoclonal Mouse IgG1 kappa Clone # MRK16
Format
Azide and BSA Free
Concentration
1 mg/ml
Product Specifications
Immunogen
Drug resistant K-562 cells.
Specificity
Recognizes a discontinuous extracellular epitope on P-glycoprotein.
Clonality
Monoclonal
Host
Mouse
Isotype
IgG1 kappa
Scientific Data Images for MDR1/ABCB1 Antibody (MRK16) - Azide and BSA Free
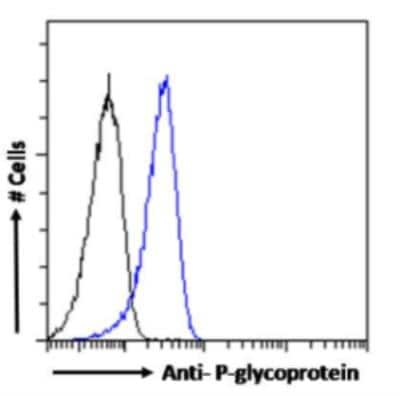
Flow Cytometry: MDR1/ABCB1 Antibody (MRK16) [NBP2-52701]
Applications for MDR1/ABCB1 Antibody (MRK16) - Azide and BSA Free
Application
Recommended Usage
Flow Cytometry
1:10 - 1:1000
Immunoprecipitation
1:10 - 1:500
Application Notes
This antibody is Cytof ready.
Formulation, Preparation, and Storage
Purification
Protein A purified
Formulation
PBS
Format
Azide and BSA Free
Preservative
0.02% Proclin 300
Concentration
1 mg/ml
Shipping
The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below.
Stability & Storage
Store at 4C for up to 3 months. For longer storage, aliquot and store at -20C.
Background: MDR1/ABCB1
Long Name
ATP-binding Cassette, Sub-family B (MDR/TAP), Member 1B
Alternate Names
ABC20, ABCB1, ABCB1B, CD243, CLCS, GP170, IBD13, PGY1
Gene Symbol
ABCB1
Additional MDR1/ABCB1 Products
Product Documents for MDR1/ABCB1 Antibody (MRK16) - Azide and BSA Free
Product Specific Notices for MDR1/ABCB1 Antibody (MRK16) - Azide and BSA Free
This product is for research use only and is not approved for use in humans or in clinical diagnosis. Primary Antibodies are guaranteed for 1 year from date of receipt.
Loading...
Loading...
Loading...
Loading...
Loading...