Mouse CX3CL1/Fractalkine Chemokine Domain Antibody
R&D Systems, part of Bio-Techne | Catalog # AF472


Key Product Details
Validated by
Biological Validation
Species Reactivity
Validated:
Mouse
Cited:
Mouse
Applications
Validated:
Neutralization, Western Blot
Cited:
Bioassay, Flow Cytometry, Immunocytochemistry, Immunohistochemistry, Immunohistochemistry-Paraffin, In vivo assay, Neutralization, Western Blot
Label
Unconjugated
Antibody Source
Polyclonal Goat IgG
Product Specifications
Immunogen
E. coli-derived recombinant mouse CX3CL1/Fractalkine
Leu22-Lys105
Accession # O35188
Leu22-Lys105
Accession # O35188
Specificity
Detects mouse Fractalkine in direct ELISAs and Western blots. In direct ELISAs, less than 40% cross-reactivity with recombinant human Fractalkine and recombinant rat Fractalkine is observed.
Clonality
Polyclonal
Host
Goat
Isotype
IgG
Endotoxin Level
<0.10 EU per 1 μg of the antibody by the LAL method.
Scientific Data Images for Mouse CX3CL1/Fractalkine Chemokine Domain Antibody
Chemotaxis Induced by CX3CL1/Fractalkine and Neutralization by Mouse CX3CL1/Fractalkine Antibody.
Recombinant Mouse CX3CL1/Fractalkine (Catalog # 571-MF) chemoattracts the BaF3 mouse pro-B cell line transfected with human CX3CR1 in a dose-dependent manner (orange line). The amount of cells that migrated through to the lower chemotaxis chamber was measured by Resazurin (Catalog # AR002). Chemotaxis elicited by Recombinant Mouse CX3CL1/Fractalkine (30 ng/mL) is neutralized (green line) by increasing concentrations of Goat Anti-Mouse CX3CL1/Fractalkine Chemokine Domain Antigen Affinity-purified Polyclonal Antibody (Catalog # AF472). The ND50 is typically 0.3-1.5 µg/mL.Detection of Mouse CX3CL1/Fractalkine by Western Blot
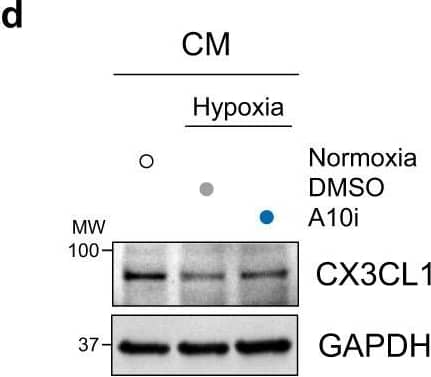
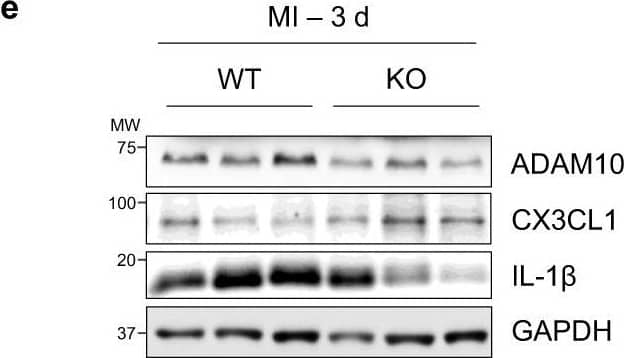
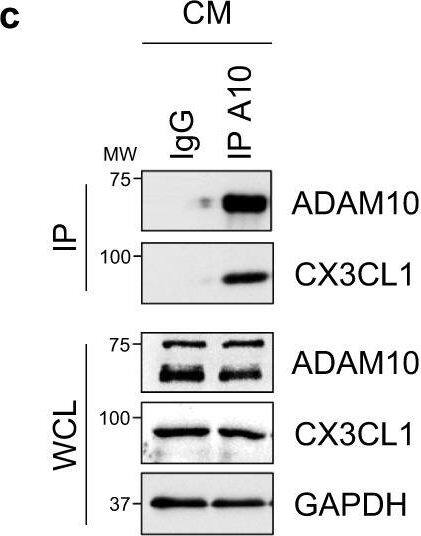
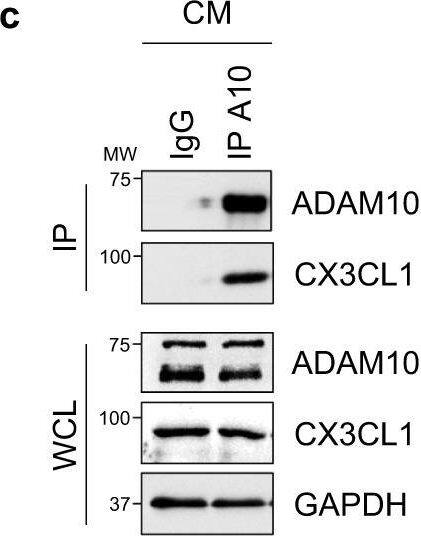
Cardiomyocyte-specific ADAM10/CX3CL1 signaling regulates neutrophil migration.a Immunofluorescence images and b quantification of CX3CL1, cardiac Troponin T (cTnT, cardiomyocytes, CM) and CD31 (endothelial cells, EC) stained heart tissue sections of sham-operated (Sham) and LAD-ligated (MI) mice 14 days after surgery. Nuclei are stained with DAPI. Representative images are shown. Scale bar, 20 µm. n = 4 per group, mean ± SEM, ns not significant, two-tailed Mann–Whitney test with Dunn’s posttest. c Co-immunoprecipitation of endogenous ADAM10 (A10) and CX3CL1 from whole-cell lysates of HL-1 cells. Representative western blots are shown (n = 4). IgG, immunoglobulin G as a control. WCL, whole-cell lysate. d Western blot analysis of CX3CL1 and e quantification of CX3CL1 expression (n = 6 per group, mean ± SEM, ns not significant, **P < 0.01, ***P < 0.001, one-way ANOVA with Tukey’s posttest) as well as f ectodomain shedding (CX3CL1 levels in supernatant) in normoxic as well as GI254023X (A10i) and DMSO treated hypoxic (1% O2) mouse cardiomyocytes (HL-1) (n = 3 per group, mean ± SEM, ns not significant, **P < 0.01, Kruskal–Wallis test with Dunn’s posttest, exact P-values are provided in the Source Data file). g Treatment scheme. HL-1 cells were cultured for 3 h under normoxic or hypoxic conditions in parallel to A10i or DMSO treatment in combination with unspecific control siRNA (siC) or CX3CL1 depletion (siCX3CL1). Supernatants were used in the lower chamber and 3 × 105 freshly isolated mouse bone marrow derived neutrophils or macrophages were seeded in the upper chamber of transwell plates (8 µm pore size) to determine migration capacity. The scheme was created using Servier Medical Art (available online: https://smart.servier.com). h Western blot analysis and i quantification of CX3CL1 expression in HL-1 cells treated for 48 h with unspecific control siRNA (siC) or CX3CL1-specific siRNA (siCX3#1 and siCX3#2). Representative western blots (n = 3 per group, mean ± SEM, **P < 0.01, Kruskal–Wallis test with Dunn’s posttest) are shown. Exact P-values: siC vs. siCX3#1, 0.0043. siC vs. siCX3#2, 0.005. Transwell migration assays of bone marrow derived j neutrophils and k macrophages using the supernatants of HL-1 cells that were cultured under hypoxic conditions in parallel to A10i or DMSO treatment in combination with unspecific control siRNA (siC) or CX3CL1 depletion (siCX3#1 and CX3#2) as chemoattractant (n = 3 per group, mean ± SEM, ns not significant, **P < 0.01, Kruskal–Wallis test with Dunn’s posttest). Source data and exact P-values are provided in the Source Data file. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36496449), licensed under a CC-BY license. Not internally tested by R&D Systems.Detection of Mouse CX3CL1/Fractalkine by Western Blot
Cardiomyocyte-specific ADAM10/CX3CL1 signaling regulates neutrophil migration.a Immunofluorescence images and b quantification of CX3CL1, cardiac Troponin T (cTnT, cardiomyocytes, CM) and CD31 (endothelial cells, EC) stained heart tissue sections of sham-operated (Sham) and LAD-ligated (MI) mice 14 days after surgery. Nuclei are stained with DAPI. Representative images are shown. Scale bar, 20 µm. n = 4 per group, mean ± SEM, ns not significant, two-tailed Mann–Whitney test with Dunn’s posttest. c Co-immunoprecipitation of endogenous ADAM10 (A10) and CX3CL1 from whole-cell lysates of HL-1 cells. Representative western blots are shown (n = 4). IgG, immunoglobulin G as a control. WCL, whole-cell lysate. d Western blot analysis of CX3CL1 and e quantification of CX3CL1 expression (n = 6 per group, mean ± SEM, ns not significant, **P < 0.01, ***P < 0.001, one-way ANOVA with Tukey’s posttest) as well as f ectodomain shedding (CX3CL1 levels in supernatant) in normoxic as well as GI254023X (A10i) and DMSO treated hypoxic (1% O2) mouse cardiomyocytes (HL-1) (n = 3 per group, mean ± SEM, ns not significant, **P < 0.01, Kruskal–Wallis test with Dunn’s posttest, exact P-values are provided in the Source Data file). g Treatment scheme. HL-1 cells were cultured for 3 h under normoxic or hypoxic conditions in parallel to A10i or DMSO treatment in combination with unspecific control siRNA (siC) or CX3CL1 depletion (siCX3CL1). Supernatants were used in the lower chamber and 3 × 105 freshly isolated mouse bone marrow derived neutrophils or macrophages were seeded in the upper chamber of transwell plates (8 µm pore size) to determine migration capacity. The scheme was created using Servier Medical Art (available online: https://smart.servier.com). h Western blot analysis and i quantification of CX3CL1 expression in HL-1 cells treated for 48 h with unspecific control siRNA (siC) or CX3CL1-specific siRNA (siCX3#1 and siCX3#2). Representative western blots (n = 3 per group, mean ± SEM, **P < 0.01, Kruskal–Wallis test with Dunn’s posttest) are shown. Exact P-values: siC vs. siCX3#1, 0.0043. siC vs. siCX3#2, 0.005. Transwell migration assays of bone marrow derived j neutrophils and k macrophages using the supernatants of HL-1 cells that were cultured under hypoxic conditions in parallel to A10i or DMSO treatment in combination with unspecific control siRNA (siC) or CX3CL1 depletion (siCX3#1 and CX3#2) as chemoattractant (n = 3 per group, mean ± SEM, ns not significant, **P < 0.01, Kruskal–Wallis test with Dunn’s posttest). Source data and exact P-values are provided in the Source Data file. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36496449), licensed under a CC-BY license. Not internally tested by R&D Systems.Applications for Mouse CX3CL1/Fractalkine Chemokine Domain Antibody
Application
Recommended Usage
Western Blot
0.1 µg/mL
Sample: Recombinant Mouse CX3CL1/Fractalkine Full Length
Sample: Recombinant Mouse CX3CL1/Fractalkine Full Length
Neutralization
Measured by its ability to neutralize CX3CL1/Fractalkine-induced chemotaxis in the BaF3 mouse pro-B cell line transfected with human CX3CR1. The Neutralization Dose (ND50) is typically 0.3-1.5 µg/mL in the presence of 30 ng/mL Recombinant Mouse CX3CL1/Fractalkine aa 25-105.
Formulation, Preparation, and Storage
Purification
Antigen Affinity-purified
Reconstitution
Reconstitute at 0.2 mg/mL in sterile PBS. For liquid material, refer to CoA for concentration.
Formulation
Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. *Small pack size (SP) is supplied either lyophilized or as a 0.2 µm filtered solution in PBS.
Shipping
Lyophilized product is shipped at ambient temperature. Liquid small pack size (-SP) is shipped with polar packs. Upon receipt, store immediately at the temperature recommended below.
Stability & Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: CX3CL1/Fractalkine
Alternate Names
FKN, Fractalkine, Neurotactin
Gene Symbol
CX3CL1
UniProt
Additional CX3CL1/Fractalkine Products
Product Documents for Mouse CX3CL1/Fractalkine Chemokine Domain Antibody
Product Specific Notices for Mouse CX3CL1/Fractalkine Chemokine Domain Antibody
For research use only
Loading...
Loading...
Loading...
Loading...