CTLA-4 Antibody Pair [HRP]
Novus Biologicals, part of Bio-Techne | Catalog # NBP2-79349

Key Product Details
Assay Type
Sandwich ELISA
Assay Range
70.31-4500 pg/ml (example only; lot dependent)
Sensitivity
70.31 pg/ml (example only; lot dependent)
Reactivity
Human
Product Specifications
Description
Solid Phase sandwich ELISA for the quantitative determination of Human CTLA-4.
Sample Volume Required
100 ul
Conjugate
HRP
Scientific Data Images for CTLA-4 Antibody Pair [HRP]
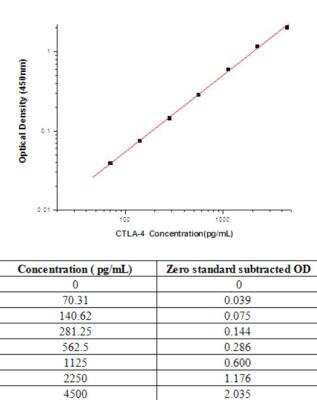
Sandwich ELISA: CTLA-4 Antibody Pair [HRP] [NBP2-79349] - This standard curve is only for demonstration purposes. A standard curve should be generated for each assay.
Kit Contents for CTLA-4 Antibody Pair [HRP]
- Mouse Monoclonal Capture Antibody
- Rabbit Polyclonal Detection Antibody (HRP-conjugated)
- Standard
Preparation and Storage
Shipping
The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below.
Stability & Storage
Storage of components varies. See protocol for specific instructions.
Background: CTLA-4
Similar to programmed cell death protein 1 (PD-1), CTLA-4 is an inhibitory immune checkpoint protein (3,5). Checkpoint blockade immunotherapy using drugs or antibodies to target CTLA-4 is one of the main approaches for cancer treatment (5). A number of drugs targeting CTLA-4, or a combination of CTLA-4/PD-1, have been approved for treatment of various cancers like melanoma, renal cell carcinoma, and colorectal cancer (5). While blocking CTLA-4 in the tumor microenvironment is a promising cancer therapeutic, the absence of CTLA-4 under normal conditions can have deleterious effects. Studies have found that patients with CTLA-4 deficiency or mutations have clinical features associated with autoimmunity and immune dysregulation (4). Treatment options for CTLA-4 deficiency includes immunoglobulin-replacement therapy, corticosteroids, CTLA-4-immunoglobulin (Ig) fusion protein, and, in life-threatening cases, hematopoietic stem cell transplantation (4,6). Additionally, engaging CD80/CD86 with CTLA-4-Ig is a common immunosuppressive treatment for rheumatoid arthritis and kidney transplant recipients (6).
References
1. Romo-Tena, J., Gomez-Martin, D., & Alcocer-Varela, J. (2013). CTLA-4 and autoimmunity: new insights into the dual regulator of tolerance. Autoimmunity reviews, 12(12), 1171-1176. https://doi.org/10.1016/j.autrev.2013.07.002
2. Hosseini, A., Gharibi, T., Marofi, F., Babaloo, Z., & Baradaran, B. (2020). CTLA-4: From mechanism to autoimmune therapy. International immunopharmacology, 80, 106221. https://doi.org/10.1016/j.intimp.2020.106221
3. Rowshanravan, B., Halliday, N., & Sansom, D. M. (2018). CTLA-4: a moving target in immunotherapy. Blood, 131(1), 58-67. https://doi.org/10.1182/blood-2017-06-741033
4. Verma, N., Burns, S. O., Walker, L., & Sansom, D. M. (2017). Immune deficiency and autoimmunity in patients with CTLA-4 (CD152) mutations. Clinical and experimental immunology, 190(1), 1-7. https://doi.org/10.1111/cei.12997
5. Rotte A. (2019). Combination of CTLA-4 and PD-1 blockers for treatment of cancer. Journal of experimental & clinical cancer research : CR, 38(1), 255. https://doi.org/10.1186/s13046-019-1259-z
6. Bluestone, J. A., St Clair, E. W., & Turka, L. A. (2006). CTLA4Ig: bridging the basic immunology with clinical application. Immunity, 24(3), 233-238. https://doi.org/10.1016/j.immuni.2006.03.001
Long Name
Cytotoxic T-lymphocyte-associated Molecule 4
Alternate Names
CD152, CTLA4
Gene Symbol
CTLA4
Additional CTLA-4 Products
Product Documents for CTLA-4 Antibody Pair [HRP]
Product Specific Notices for CTLA-4 Antibody Pair [HRP]
This product is for research use only and is not approved for use in humans or in clinical diagnosis. Antibody Pairs are guaranteed for 6 months from date of receipt.
Loading...
Loading...
Loading...
Loading...