Human Cyclophilin-F ELISA Kit (Colorimetric)
Novus Biologicals, part of Bio-Techne | Catalog # NBP2-60646

Key Product Details
Sample Type & Volume Required Per Well
Serum, Plasma, Cell Culture
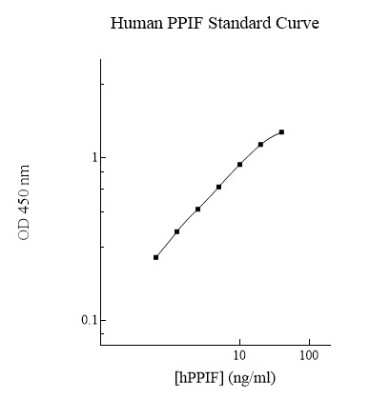
Sensitivity
1.4 ng/ml (example only; lot dependent)
Assay Range
0.625 - 40 ng/mL (example only; lot dependent)
Product Specifications
Assay Type
Sandwich ELISA
Kit Type
ELISA Kit (Colorimetric)
Reactivity
Human
Description
This assay employs a quantitative enzyme immunoassay technique that measures the specified antigen in samples.
Precision
Intra-Assay Precision (Precision within an assay) CV% < 3.90% (example only; lot dependent)
Inter-Assay Precision (Precision between assays) CV% < 7.40% (example only; lot dependent)
Recovery for Human Cyclophilin-F ELISA Kit (Colorimetric)
Recovery
97.00% (example only; lot dependent)
Scientific Data Images for Human Cyclophilin-F ELISA Kit (Colorimetric)
ELISA: Human Cyclophilin-F ELISA Kit (Colorimetric) [NBP2-60646]
Preparation and Storage
Shipping
The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below.
Stability & Storage
Storage is content dependent.
Background: Cyclophilin-F
Alternate Names
Cyclophilin F, CYP3FLJ90798, Cyp-D, hCyP3, MGC117207, mitochondrial, peptidyl-prolyl cis-trans isomerase, mitochondrial, peptidylprolyl isomerase F, peptidylprolyl isomerase F (cyclophilin F)
Gene Symbol
PPIF
Additional Cyclophilin-F Products
Product Documents for Human Cyclophilin-F ELISA Kit (Colorimetric)
Product Specific Notices for Human Cyclophilin-F ELISA Kit (Colorimetric)
This product is for research use only and is not approved for use in humans or in clinical diagnosis. ELISA Kits are guaranteed for 6 months from date of receipt.
Loading...
Loading...
Loading...
Loading...