| Key Benefits: |
Learn more about Fluorescent Glycan Labeling and Detection
Summary for PNGase F N-glycan Releasing Kit
The PNGase F N-glycan Releasing Kit (Catalog # EA006) provides PNGase F and necessary buffers for gel analysis as well as mass spectrometry analysis. The receptor binding domain of SARS-CoV-2 spike protein that is fluorescently labeled on N-glycans is provided as a convenient control, allowing for monitoring of the deglycosylation process in gel through fluorescent imaging. PNGase F N-glycan Releasing Kit Features - Gel and mass spectrometry analysis of N‑glycans
- Cy5-RBD fluorescent control to monitor the deglycosylation process through fluorescent imaging via gel electrophoresis
- Protocols are provided to guide further assay optimization
- Removes N-linked glycans from Glycoproteins
- Guaranteed Enzymatic Activity: Enzymatic activity tested in a deglycosylation assay
Kit Contents Store the unopened kit at ≤ -70 °C. Do not use past kit expiration date. - PNGase F Protein
- Cy5-Neu5Ac Labeled RBD Protein (Cy5-RBD)
- PNGase F Assay Buffer
- Denaturing Buffer
- Renaturing Buffer
- 6X SDS Gel Loading Dye
Other Reagents Required - 37°C incubator
- Microcentrifuge tubes or equivalent
- Pipettes and pipette tips
- Deionized or distilled water
- Equipment to run SDS-PAGE gel
- Fluorescent imager
- Mass Spectrometry Instrumentation
Preparation and Storage Stability & Storage
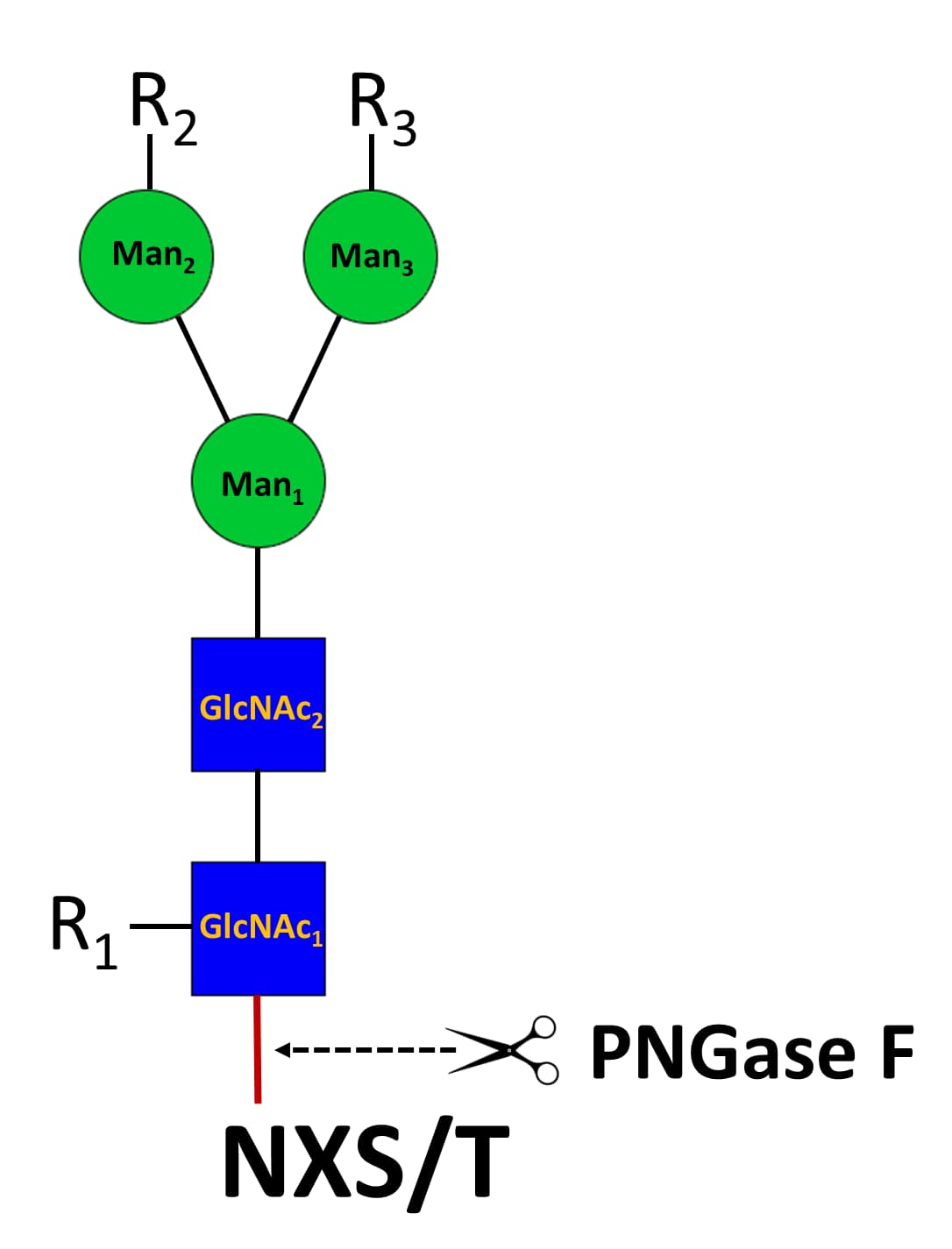
Store the unopened product at ≤ -70 °C. Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Do not use past expiration date. Background: PNGase F N-Glycosylation found on the asparagine residue of the sequon NXT/S is one of the most commonposttranslational modifications of proteins in eukaryotic cells. N‑glycans are initiated as high-mannose glycan, which are converted to hybrid and complex N‑glycans during the maturation process in the Golgi apparatus. N‑glycosylation involves in myriad of biological functions such as receptor binding, cell signaling, immune recognition, inflammation, and pathogenicity. To study these N-glycans, it is essential to release them from proteins. PNGase F is a peptide N‑glycosidase F from Flavobacterium meningosepticum and the most used enzyme to extensively release various N‑glycans, including high-mannose, hybrid and complex types of N‑Glycans, except those N‑glycans with the innermost GlcNAc modified with a core‑3 linked fucose. PNGase F digestion results in the release of entire N‑glycans and conversion of the underlying asparagine residue (N) to an aspartic residue (D), allowing identification of the glycosylation site by mass spectrometry.
Product Specifications for PNGase F N-glycan Releasing Kit
| Species |
Multi-Species
|
Scientific Data Examples for PNGase F N-glycan Releasing Kit
|
|
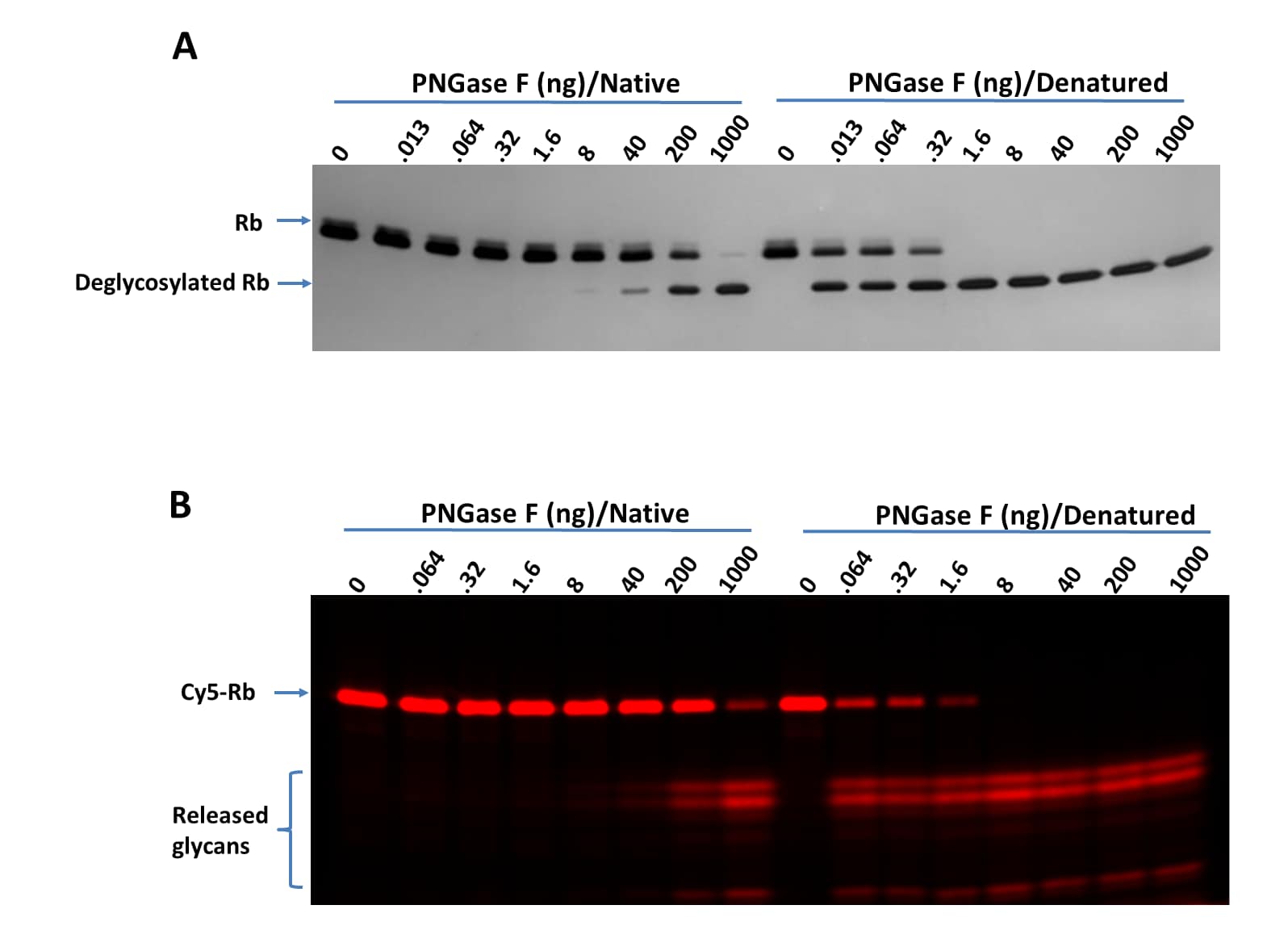
Titration of PNGase F on RNase B (Rb) and Cy5-Labeled RNase B (Cy5-Rb) using bothnative and denaturing assay protocols.
Deglycosylation of Rb (A) and Cy5-Rb (B) with PNGase F N-glycan Releasing Kit(Catalog # EA006) using both the native and denaturing assay protocols. >95%deglycosylation was achieved with 1000 ng and 1.6 ng of PNGase F under native and denaturingconditions, respectively. Digested samples were separated on 17% SDS gel. A. Rb (1 μg perdigestion) was digested with indicated amounts of PNGase F and imaged with silver staining.B. Cy5-Rb (0.2 μg for digestion) was digested with indicated amounts of PNGase F and visualized with fluorescent imaging. Labeling on RNase B was introduced by serial treatment with Recombinant HumanN-Acetylglucosaminyltransferase1/MGAT1 (8334-GT), UDP-GlcNAc,Recombinant Human B4GalT1 Protein (3609-GT), UDP-Gal, Recombinant HumanST6GAL1 (aa44-406) Protein (7620-GT), CMP-Cy5-Sialic Acid (ES302)(Wu, Z. et al. (2020) Glycobiology 30:970). Denaturing increased the PNGase F sensitivity by 500-fold.
|
|
|
Titration of PNGase F on Cy5-Neu5Ac Labeled RBD Protein (Cy5-RBD) using both native and denaturing assay protocols.
Deglycosylation of Cy5-RBD with PNGase F N-glycan Releasing Kit (Catalog # EA006) using both the native and denaturing assay protocols. Cy5-RBD (1 µg per digestion) was treated with indicated amounts of PNGase F in 20 µL under both denaturing and native conditions at 37 °C for 60 minutes then separated on 17% SDS gel and imaged by trichloroethanol (TCE) staining (top panel, only proteins were visible, and the lower portion wascropped out) and fluorescent imaging (lower panel). >95% deglycosylation was achieved with 200 ng and 1.6 ng of PNGase F under native and denaturing conditions, respectively.
|
|
|
Titration of PNGase F on Cy5-Neu5Ac Labeled ACE-2 (Cy5-ACE-2) using both native and denaturing assay protocols.
Deglycosylation of Cy5-ACE-2 with PNGase F N-glycan Releasing Kit (Catalog # EA006) using both the native and denaturing assay protocols Cy5-ACE-2. (1 µg for each digestion) was treated with indicated amounts of PNGase F in 20 µL under both denaturing and native conditions at 37 °C for 30 minutes then separated on 17% SDS gel and imaged by TCE staining (top panel, only proteins were visible, and the lower portion was cropped out) and fluorescent imaging (lower panel). Multiple bands are visible for the released glycans suggesting the complicated nature of the glycosylation on ACE-2. >90% deglycosylation was achieved with 2000 ng and 0.2 ng of PNGase F under native and denaturing conditions, respectively.
|
|
|
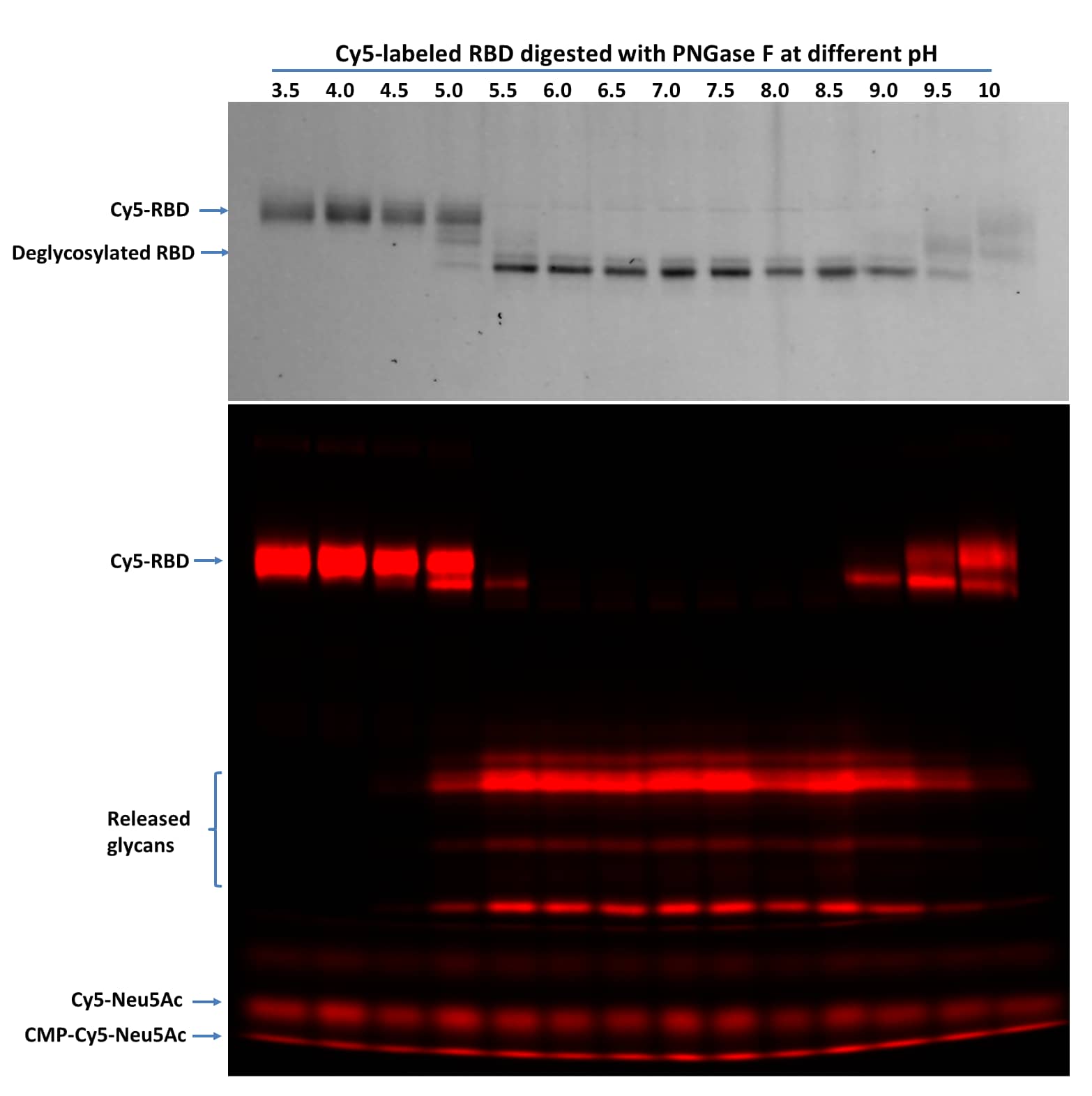
PNGase F pH profile test on Cy5-Neu5Ac Labeled RBD Protein (Cy5-RBD) using denaturing assay protocols.
Denatured Cy5-RBD (1 µg per digestion) was treated with 5 ng of PNGase F at indicated pH in 20 µL at 37 °C for 60 minutes then separated on 17% SDS gel and imaged by TCE staining (top panel, only proteins were visible, and the lower portion was cropped out) and fluorescent imaging (lower panel). PNGase F showed activity from pH 5.0 to 10 with complete glycan removal between pH 6.0 to pH 8.5.
|
|
|
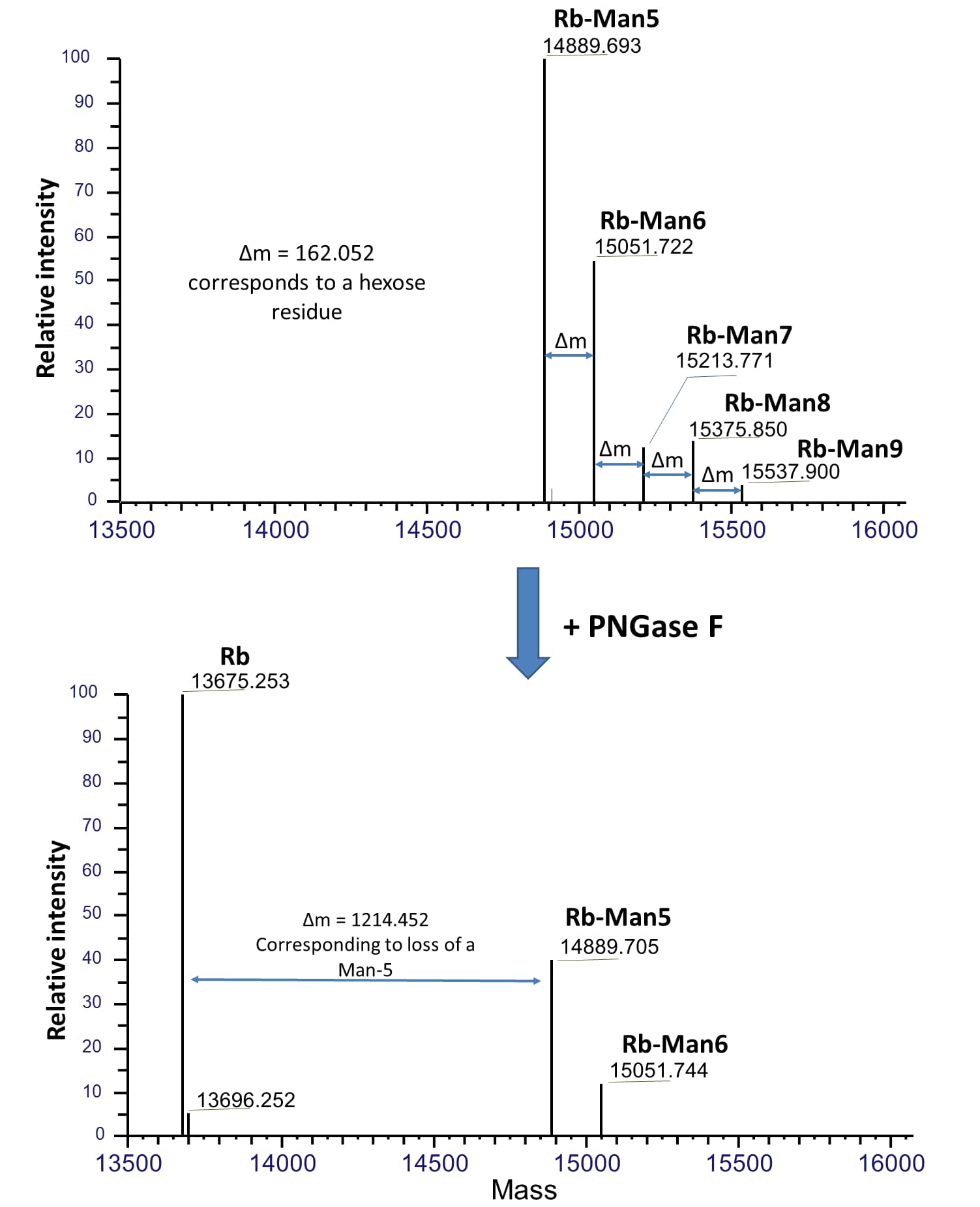
Mass spectrometry analysis of PNGase F treated bovine fetal RNase B.
Native RNase B of different glycoforms with high-mannose glycans, Man-5 to Man-9, are indicated as Rb-Man5 to Rb-Man9 respectively. Upon PNGase F treatment under native condition, majority of these glycans were removed and resulted in deglycosylated RNase B (Rb). Mass spectrometry was performed using a Thermo Scientific Q Exactive HF system connected to a Thermo Vanquish LC. Samples were separated on a C4 column then analyzed using full scan at 240,000 resolution in intact mode. Data was analyzed using Thermo BioPharma Finder software.
|
Preparation & Storage
| Storage |
Store the unopened product at -70 °C. Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Do not use past expiration date.
|
Assay Procedure
ASSAY PROTOCOL Denaturing Assay Protocol Reaction volumes can be increased or decreased proportionally. It is recommended to start with concentrated samples so that less sample volume is required, therefore less denaturing buffer will be applied to final reactions to minimize the negative effects of SDS on PNGase F. PNGase F can be directly used or diluted in 1X PNGase F Assay Buffer before usage. A titration on PNGase F maybe performed to determine amount of enzyme is needed for a specific sample protein. For negative controls, replace PNGase F with 1X Assay Buffer. Make sure to mix well all components in each step. Reagent Preparation
Prepare 1X Assay Buffer by diluting the 10X PNGase F Assay Buffer with deionized or distilled water. - Sample Preparation
- In a centrifuge tube add the following items stepwise, 10 µL Sample Protein or Cy5-RBD and 1 µL Denaturing Buffer (10X).
- Heat at 95°C for 5 minutes and then chill on ice. Briefly centrifuge condensation.
- Add 1 µL Renaturing Buffer (10X)
Note: The sample protein may be diluted with 1X PNGase F Assay Buffer to a desired concentration here. - PNGase F Digestion
- Add 5 µL of Denatured Sample Protein or Cy5-RBD, 14 µL of 1X Assay Buffer, and 1 µL PNGase F to a new centrifuge tube.
- Incubate at 37 °C for 60 minutes to overnight.
- Add 4 µL of 6X SDS Gel Loading Buffer to the tube.
- SDS-Gel Separation and Imaging
- Load half of the final digestion volume to an SDS Gel.
- Run the gel until the dye front reaches the end of the gel.
- Perform fluorescent imaging first if Cy5-RBD is used as a control.
- Perform regular protein imaging via Silver, TCE or Coomassie® Blue staining.
NATIVE ASSAY PROTOCOL
Native assay protocol is specifically for samples that are intended for mass spec analysis. Reaction volumes can be increased proportionally. Proteins under native conditions are much more resistant to PNGase F digestion. To completely deglycosylate proteins under native conditions, high concentration PNGase F and longer incubation time are recommended.
Customer Reviews for PNGase F N-glycan Releasing Kit
Have you used PNGase F N-glycan Releasing Kit?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
Product Documents for PNGase F N-glycan Releasing Kit
|