Recombinant Human BFAR GST (N-Term) Protein
Novus Biologicals, part of Bio-Techne | Catalog # H00051283-P01

Key Product Details
Source
Tag
Conjugate
Applications
Product Specifications
Description
Source: Wheat Germ (in vitro)
Amino Acid Sequence: MEEPQKSYVNTMDLERDEPLKSTGPQISVSEFSCHCCYDILVNPTTLNCGHSFCRHCLALWWASSKKTECPECREKWEGFPKVSILLRDAIEKLFPDAIRLRFEDIQQNNDIVQSLAAFQKYGNDQIPLAPNTGRANQQMGGGFFSGVLTALTGVAVVLLVYHWSSRESEHDLLVHKAVAKWTAEEVVLWLEQLGPWASLYRERFLSERVNGRLLLTLTEEEFSKTPYTIENSSHRRAILMELERVKALGVKPPQNLWEYKAVNPGRSLFLLYALKSSPRLSLLYLYLFDYTDTFLPFIHTICPLQEDSSGEDIVTKLLDLKEPTWKQWREFLVKYSFLPYQLIAEFAWDWLEVHYWTSRFLIINAMLLSVLELFSFWRIWSRSELKTVPQRMWSHFWKVSTQGLFVAMFWPLIPQFVCNCLFYWALYFNPIINIDLVVKELRRLETQVL
Purity
Predicted Molecular Mass
Disclaimer note: The observed molecular weight of the protein may vary from the listed predicted molecular weight due to post translational modifications, post translation cleavages, relative charges, and other experimental factors.
Activity
Protein / Peptide Type
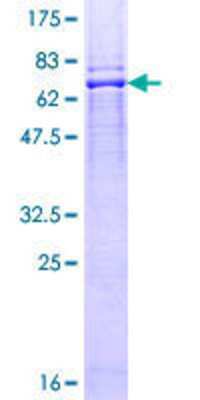
Scientific Data Images for Recombinant Human BFAR GST (N-Term) Protein
Formulation, Preparation and Storage
H00051283-P01
| Preparation Method | in vitro wheat germ expression system |
| Formulation | 50 mM Tris-HCl, 10 mM reduced Glutathione, pH 8.0 in the elution buffer. |
| Preservative | No Preservative |
| Concentration | Please see the vial label for concentration. If unlisted please contact technical services. |
| Shipping | The product is shipped with dry ice or equivalent. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Store at -80C. Avoid freeze-thaw cycles. |
Background: BFAR
Alternate Names
Gene Symbol
Additional BFAR Products
Product Documents for Recombinant Human BFAR GST (N-Term) Protein
Product Specific Notices for Recombinant Human BFAR GST (N-Term) Protein
This product is produced by and distributed for Abnova, a company based in Taiwan.
This product is for research use only and is not approved for use in humans or in clinical diagnosis. This product is guaranteed for 1 year from date of receipt.