Recombinant Human EGLN1/PHD2 GST (N-Term) Protein
Novus Biologicals, part of Bio-Techne | Catalog # H00054583-P01

Key Product Details
Source
Wheat germ
Tag
GST (N-Term)
Conjugate
Unconjugated
Applications
ELISA, Enzyme Activity, Affinity Purification, Functional Assay, Microarray, Western Blot
Product Specifications
Description
A recombinant protein with GST tag at N-terminal corresponding to the amino acids 1-128 of Human EGLN1.
Source: Wheat Germ (in vitro)
Amino Acid Sequence: MVACYPGNGTGYVRHVDNPNGDGRCVTCIYYLNKDWDAKVSGGILRIFPEGKAQFADIEPKFDRLLFFWSDRRNPHEVQPAYATRYAITVWYFDADERARAKVKYLTGEKGVRVELNKPSDSVGKDVF
Purity
>80% by SDS-PAGE and Coomassie blue staining
Predicted Molecular Mass
41 kDa.
Disclaimer note: The observed molecular weight of the protein may vary from the listed predicted molecular weight due to post translational modifications, post translation cleavages, relative charges, and other experimental factors.
Disclaimer note: The observed molecular weight of the protein may vary from the listed predicted molecular weight due to post translational modifications, post translation cleavages, relative charges, and other experimental factors.
Activity
This protein was produced in an in vitro wheat germ expression system that should preserve correct conformational folding that is necessary for biological function. While it is possible that this protein could display some level of activity, the functionality of this protein has not been explicitly measured or validated.
Protein / Peptide Type
Recombinant Protein
Scientific Data Images for Recombinant Human EGLN1/PHD2 GST (N-Term) Protein
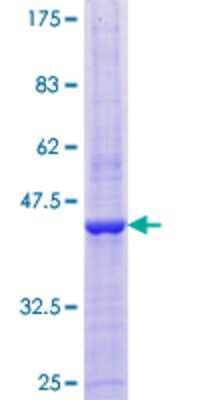
Recombinant Human EGLN1/PHD2 GST (N-Term) Protein [H00054583-P01] - Analysis by 12.5% SDS-PAGE. Gel stained with Coomassie Blue.
Formulation, Preparation and Storage
H00054583-P01
| Preparation Method | In vitro wheat germ expression system |
| Formulation | Elution buffer: 50 mM Tris-HCl, 10 mM reduced Glutathione, pH 8.0 |
| Preservative | No Preservative |
| Concentration | Please see the vial label for concentration. If unlisted please contact technical services. |
| Shipping | The product is shipped with dry ice or equivalent. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Store at -80C. Avoid freeze-thaw cycles. |
Background: EGLN1/PHD2
EGLN1/PHD2 has been implicated in several critical processes including erythropoiesis, angiogenesis, and metabolism as well as various pathologies such as cancer (2, 5, 6). Studies in mice have found that somatic deletion of PHD2 resulted in higher vascular endothelial growth factor A (VEGF-A) levels, increased blood vessel formation, and more erythropoietin (EPO), leading to severe polycythemia or erythrocytosis (high red blood cell (RBC) volume) (6). Another study revealed that specific point mutations in EGLN1/PHD2 led to elevated EPO and RBC mass associated with hemorrhages and strokes (6). Accordingly, given the known role of PHD2 in inhibition of EPO production, PHD2 inhibitors are being studied as a potential therapeutic for anemia (6). Additionally, dysregulation in EGLN1, and specifically the PHD2-VHL-HIF-1alpha pathway, has been associated with the development of pheochromocytomas (PCC) and sympathetic paragangliomas (PGL), which are rare neuroendocrine tumors (2). Besides pathological features, EGLN1/PHD2 may also be important for high altitude adaptation as two coding sequence variants in PHD2 are prevalent in the Tibetan population but is very rare in people at lower altitudes (2).
Alternate names for EGLN1/PHD2 include HIF Prolyl Hydroxylase 2, PH2, Prolyl hydroxylase domain containing protein 2, HIF2PH2, HIF-Prolyl hydroxylase 2, egl nine homolog 1, and C1orf12.
References
1. Amorim-Pires, D., Peixoto, J., & Lima, J. (2016). Hypoxia Pathway Mutations in Pheochromocytomas and Paragangliomas. Cytogenetic and genome research. https://doi.org/10.1159/000457479
2. Gardie, B., Percy, M. J., Hoogewijs, D., Chowdhury, R., Bento, C., Arsenault, P. R., Richard, S., Almeida, H., Ewing, J., Lambert, F., McMullin, M. F., Schofield, C. J., & Lee, F. S. (2014). The role of PHD2 mutations in the pathogenesis of erythrocytosis. Hypoxia (Auckland, N.Z.). https://doi.org/10.2147/HP.S54455
3. Minervini, G., Quaglia, F., & Tosatto, S. C. (2015). Insights into the proline hydroxylase (PHD) family, molecular evolution and its impact on human health. Biochimie. https://doi.org/10.1016/j.biochi.2015.07.009
4. Semenza G. L. (2007). Hypoxia-inducible factor 1 (HIF-1) pathway. Science's STKE : signal transduction knowledge environment. https://doi.org/10.1126/stke.4072007cm8
5. Chan, D. A., & Giaccia, A. J. (2010). PHD2 in tumour angiogenesis. British journal of cancer. https://doi.org/10.1038/sj.bjc.6605682
6. Meneses, A. M., & Wielockx, B. (2016). PHD2: from hypoxia regulation to disease progression. Hypoxia (Auckland, N.Z.). https://doi.org/10.2147/HP.S53576
Long Name
Egl Nine Homolog 1/Prolyl Hydroxylase Domain-containing Protein 2
Alternate Names
C1orf12, HIFPH2, HPH2, PHD2, SM20, ZMYND6
Gene Symbol
EGLN1
Additional EGLN1/PHD2 Products
Product Documents for Recombinant Human EGLN1/PHD2 GST (N-Term) Protein
Product Specific Notices for Recombinant Human EGLN1/PHD2 GST (N-Term) Protein
This product is produced by and distributed for Abnova, a company based in Taiwan.
This product is for research use only and is not approved for use in humans or in clinical diagnosis. This product is guaranteed for 1 year from date of receipt.
Loading...
Loading...
Loading...
Loading...