Recombinant Human BMP-2 GMP Protein, CF GMP
R&D Systems, part of Bio-Techne | Catalog # 355-GMP

Key Product Details
Source
Accession #
Structure / Form
Conjugate
Applications
Product Specifications
Source
Gln283-Arg396
Manufactured and tested under current Good Manufacturing Practice (GMP) guidelines.
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
SDS-PAGE
Activity
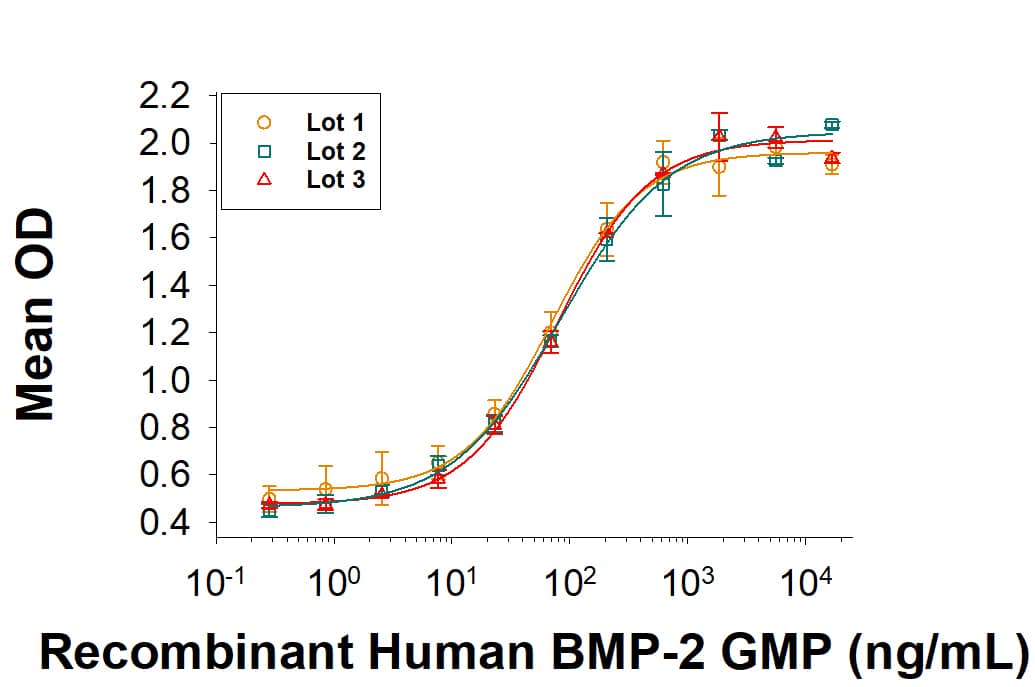
The ED50 for this effect is 40-200 ng/mL.
The specific activity of recombinant human BMP-2 is >5.0 x 105 units/mg, which is calibrated against the human BMP-2 WHO Standard (NIBSC code: 93/574).
Host Cell Protein
Mycoplasma
Scientific Data Images for Recombinant Human BMP-2 GMP Protein, CF
Recombinant Human BMP-2 GMP Protein Bioactivity
GMP-grade Recombinant Human BMP-2 (Catalog # 355-GMP) induces alkaline phosphatase production in the ATDC5 mouse chondrogenic cell line. The ED50 for this effect is 40-200 ng/mL. Three independent lots were tested for activity and plotted on the same graph to show lot-to-lot consistency of GMP BMP-2.Recombinant Human BMP-2 GMP Protein SDS-PAGE
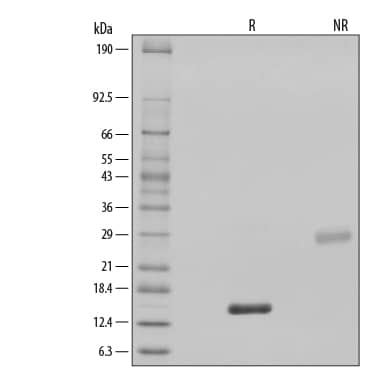
1 μg/lane of GMP-grade Recombinant Human BMP-2 (Catalog # 355-GMP) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by silver staining, showing single bands at 15 kDa and 30 kDa, respectively.Formulation, Preparation and Storage
355-GMP
| Formulation | Lyophilized from a 0.2 μm filtered solution in Glycine, Sucrose, Tween® 80 and Glutamic Acid. |
| Reconstitution | Reconstitute at 100 μg/mL in 4 mM HCl. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: BMP-2
References
- Kishigami, S. and Y. Mishina (2005) Cytokine Growth Factor Rev. 16:265.
- Chen, D. et al. (2004) Growth Factors 22:233.
- Wozney, J. et al. (1988) Science 242:1528.
- Sun, P.D. and D.R. Davies (1995) Annu. Rev. Biophys. Biomol. Struct. 24:269.
- Sebald, W. et al. (2004) Biol. Chem. 385:697.
- De Luca, F. et al. (2001) Endocrinology 142:430.
- Davidson, E.N.B., et al. (2007) Arthritis Res. Ther. 9:R102.
- Ryoo, H.-M. et al. (2006) Gene 366:51.
- Kramer, J. et al. (2000) Mech. Dev. 92:193.
- Li, X. et al. (2008) Atherosclerosis January 5 epub.
- Zhu, W. et al. (2004) J. Bone Miner. Res. 19:2021.
- Peiris, D. et al. (2007) Am. J. Physiol. Gastrointest. Liver Physiol. 292:G753.
- Kano, Y. et al. (2005) Endocrinology 146:5332.
- Hallahan, A.R. et al. (2003) Nat. Med. 9:1033.
- Wang, Y.-X. et al. (2007) Cardiovasc. Res. 74:290.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional BMP-2 Products
Product Documents for Recombinant Human BMP-2 GMP Protein, CF
Manufacturing Specifications
GMP ProteinsR&D Systems, a Bio-Techne Brand's GMP proteins are produced according to relevant sections of the following documents: USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Eu. Ph. 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products.
R&D Systems' quality focus includes:
- Manufactured and tested under an ISO 9001:2015 and ISO 13485:2016 certified quality system
- Documented processes and QA control of documentation and process changes
- Personnel training programs
- Raw material testing and vendor qualification/monitoring
- Fully validated equipment, processes and test methods
- Equipment calibration schedules using a computerized calibration program
- Facility maintenance, safety programs and pest control
- Material review process for variances
- Monitoring of stability over product shelf-life
R&D Systems strives to provide our customers with the analytical characteristics of each product so that customers may determine whether our products are appropriate for their research. The Certificate of Analysis provided contains the following lot specific information:
- N-terminal amino acid analysis, SDS-PAGE analysis, and endotoxin level (as determined by LAL assay) performed on each bulk QC lot, not on individual bottlings of each QC lot
- Post-bottling lot-specific bioassay results (compliance with an established range) and results of microbial testing according to USP
- Host Cell Protein testing performed by ELISA
- Mycoplasma testing by ribosomal RNA hybridization assay
Additional testing and documentation requested by the customer can be arranged at an additional cost.
Production records and facilities are available for examination by appropriate personnel on-site at R&D Systems in Minneapolis, Minnesota USA.
R&D Systems sells GMP grade products for preclinical or clinical ex vivo use. They are not for in vivo use. Please read the following End User Terms prior to using this product.
Product Specific Notices for Recombinant Human BMP-2 GMP Protein, CF
END USER TERMS OF USE OF PRODUCT
The following terms are offered to you upon your acceptance of these End User Terms of Use of Product. By using this product, you indicate your acknowledgment and agreement to these End User Terms of Use of Product. If you do not agree to be bound by and comply with all of the provisions of these End User Terms of Use of Product, you should contact your supplier of the product and make arrangements to return the product.
We suggest you print and retain a copy of these End User Terms of Use of Product for your records.
The End User is aware that R&D Systems, Inc. sells GMP products for preclinical or clinical ex vivo use and not for in vivo use. The End User further agrees, as a condition of the sale of R&D Systems' GMP products that: a) the End User will not use this GMP Product in any procedure wherein the product may be directly or indirectly administered to humans, unless the End User has obtained, or prior to their use will have obtained, an Investigational New Drug (IND) exemption from the FDA and will use the product only in accordance with the protocols of such IND and of the Institutional Review Board overseeing the proposed research, or b) the End User will use the products outside of the United States in accordance with the protocols of research approved by the Institutional Review Board or authorized ethics committee and regulatory agencies to which the End User is subject to in their territory.
R&D Systems, Inc. has the right, at its sole discretion, to modify, add or remove any terms or conditions of these End User Terms of Use without notice or liability to you. Any changes to these End User Terms of Use are effective immediately following the printing of such changes on this product insert. The most recent version of these End User Terms of Use of Product may be found at: RnDSystems.com/Legal.
You agree to review these End User Terms of Use of Product to ensure any subsequent use by you of R&D Systems' GMP Products following changes to these End User Terms of Use of Product constitutes your acceptance of all such changes.
TERMS AND CONDITIONS
The following limitation applies to R&D Systems' warranty and liability for damages: All products are warranted to meet R&D Systems' published specifications when used under normal laboratory conditions.
R&D SYSTEMS DOES NOT MAKE ANY OTHER WARRANTY OR REPRESENTATION WHATSOEVER, WHETHER EXPRESS OR IMPLIED, WITH RESPECT TO ITS PRODUCTS. IN PARTICULAR, R&D SYSTEMS DOES NOT MAKE ANY WARRANTY OF SUITABILITY, NONINFRINGEMENT, MERCHANTABILITY OR FITNESS FOR ANY PARTICULAR PURPOSE.
NOTWITHSTANDING ANY OTHER PROVISIONS OF THESE TERMS AND/OR ANY OTHER AGREEMENT BETWEEN R&D SYSTEMS AND PURCHASER FOR THE PURCHASE OF THE PRODUCTS, R&D SYSTEMS' TOTAL LIABILITY TO PURCHASER ARISING FROM OR IN RELATION TO THESE TERMS, AN AGREEMENT BETWEEN THE PARTIES OR THE PRODUCTS, WHETHER ARISING IN CONTRACT, TORT OR OTHERWISE SHALL BE LIMITED TO THE TOTAL AMOUNT PAID BY PURCHASER TO R&D SYSTEMS FOR THE APPLICABLE PRODUCTS. IN NO EVENT WILL R&D SYSTEMS BE LIABLE FOR THE COST OF PROCUREMENT OF SUBSTITUTE GOODS.
Full details of R&D Systems' Terms and Conditions of Sale can be found online at: RnDSystems.com/Legal.
For preclinical, or clinical ex vivo use