Recombinant Human Dystroglycan Fc Chimera Protein, CF
R&D Systems, part of Bio-Techne | Catalog # 10223-DG

Key Product Details
Source
Accession #
Structure / Form
Conjugate
Applications
Product Specifications
Source
| Human Dystroglycan (Gln28-Val749) Accession # Q14118 |
IEGRMD | Human IgG1 (Pro100-Lys330) |
| N-terminus | C-terminus |
Purity
Endotoxin Level
N-terminal Sequence Analysis
Gln313 (blocked) of alpha chain and Ser654 of beta chain
Predicted Molecular Mass
SDS-PAGE
Activity
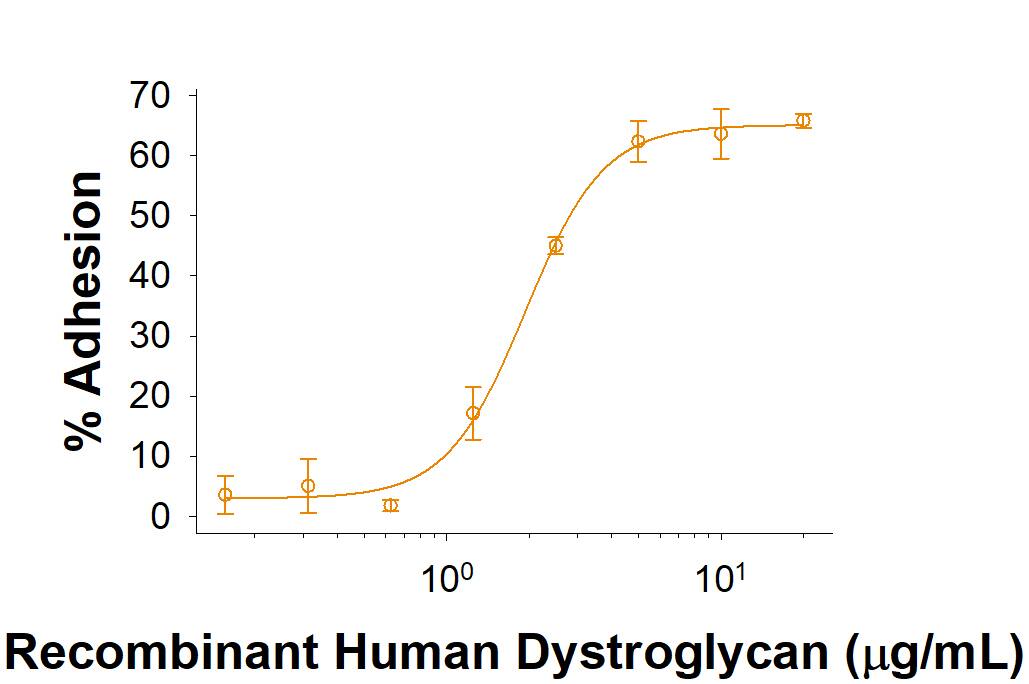
The ED50 for this effect is 1.5-9 μg/mL.
Scientific Data Images for Recombinant Human Dystroglycan Fc Chimera Protein, CF
Recombinant Human Dystroglycan Fc Chimera Protein Bioactivity
Immobilized Recombinant Human Dystroglycan Fc Chimera (Catalog # 10223-DG) enhances the adhesion of H4 human neuroglioma cells. The ED50 for this effect is 1.5-9 μg/mL.Formulation, Preparation and Storage
10223-DG
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS. |
| Reconstitution | Reconstitute at 500 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: Dystroglycan
Dystroglycan, also called DAG1 (dystrophin‑associated glycoprotein 1) or DG, is a heterodimeric adhesion molecule that links the extracellular matrix (ECM) to the cell cytoskeleton (1‑4). Human DAG1 is a type I transmembrane protein that is initially expressed as a large prepro protein. Autocatalysis of the proform produces two fragments (an alpha and beta chain) that remain noncovalently‑linked. The alpha chain (aa 28-653) contains a mucin‑like region, while the beta chain (aa) consists of an extracellular domain, a transmembrane region, and a cytoplasmic domain (5). Over aa 28-749, human DAG1 shares 93% aa sequence identity with mouse DAG1. DAG1 is widely expressed but differentially O‑glycosylated on skeletal muscle and epithelia (which contain a 160 kDa alpha ‑chain) as compared to cardiac muscle, smooth muscle, fibroblasts, keratinocytes, lymphocytes, and hematopoietic stem cells (which contain a 100‑140 kDa alpha ‑chain) (1‑3, 6‑9). DAG1 binding of ECM molecules is influenced by its alpha ‑chain O‑glycosylation (2, 6‑10). In addition to skeletal muscle and neuromuscular junctions in which DAG1 binds several ECM molecules, DAG1 is important for neuronal migration (through neurexin interactions), keratinocyte attachment to the ECM (through laminin), and adhesion at the immunological synapse and in the hematopoietic stem cell niche (through agrin) (3, 6‑11). In muscle, the beta ‑chain cytoplasmic domain connects with the cytoskeleton via formation of the dystrophin‑glycoprotein complex with isoforms of dystrophin, sarcoglycan, syntrophin, and sarcospan (3). This complex is critical for skeletal muscle viability and regeneration (3, 4, 10, 11). MMP9 cleavage of the 44 kDa beta ‑chain creates a 30 kDa transmembrane form that causes dissociation of the heterodimer and a down‑regulation of ECM interactions (6, 12). Dystroglycanopathies, a group of congenital muscular dystrophies affecting the brain, eye and skeletal muscle, are caused by either abnormalities in glycosyltransferases, or their accessory proteins, or rare DAG1 polymorphisms. All result in DAG1 hypoglycosylation, especially of O‑mannosyl forms, and affect DAG1 binding to ECM proteins (2, 3, 10, 13, 14).
References
- Ibraghimov-Bedkrovnaya, O. et al. (1993) Hum. Mol. Genet. 2:1651.
- Godfrey, C. et al. (2011) Curr. Opin. Genet. Dev. 21:278.
- Barresi, R. and Campbell K.P. (2006) J. Cell Sci. 119:199.
- Durbeej, M. and K.P. Campbell (1999) J. Biol. Chem. 274:26609.
- Akhavan, A. et al. (2008) FASEB J. 22:612.
- Herzog, C. et al. (2004) J. Invest. Dermatol. 122:1372.
- Leonoudakis, D. et al. (2010) J. Cell Sci. 123:3683.
- Zhang, J. et al. (2006) FASEB J. 20:50.
- Mazzon, C. et al. (2011) Blood 118:2733.
- Michele, D.E. et al. (2002) Nature 418:417.
- Cohn, R.D. et al. (2002) Cell 110:639.
- Bozzi, M. et al. (2009) IUBMB Life 61:1143.
- Yoshida-Moriguchi, T. et al. (2010) Science 327:88.
- Hara, Y. et al. (2011) N. Eng. J. Med. 364:939.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional Dystroglycan Products
Product Documents for Recombinant Human Dystroglycan Fc Chimera Protein, CF
Product Specific Notices for Recombinant Human Dystroglycan Fc Chimera Protein, CF
For research use only