Recombinant Human Fc epsilon RI alpha Avi His Protein, CF
R&D Systems, part of Bio-Techne | Catalog # AVI6678

Key Product Details
Source
Accession #
Structure / Form
Conjugate
Applications
Product Specifications
Source
| Human FCER-1 alpha (Val26-Gln205) Accession # P12319.1 |
Avi-tag | 6-His tag |
| N-terminus | C-terminus |
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
SDS-PAGE
Activity
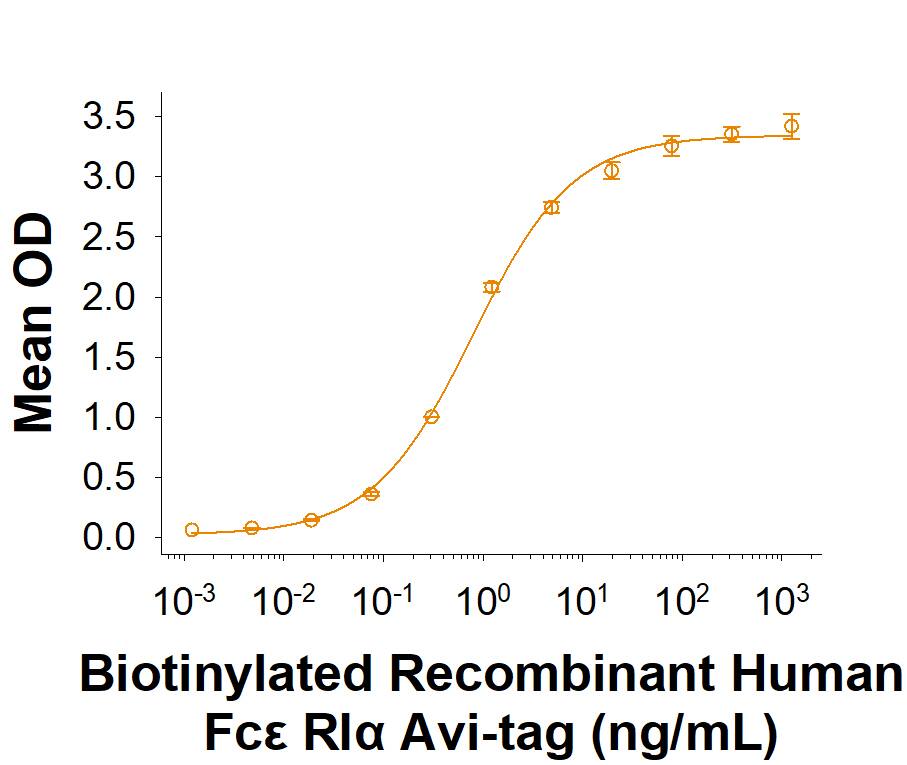
When Human IgE is immobilized at 1.00 µg/mL (100 µL/well), Biotinylated Recombinant Human Fc epsilon RI alpha Avi-tag His-tag binds with an ED50 of 0.300-1.80 ng/mL.
Scientific Data Images for Recombinant Human Fc epsilon RI alpha Avi His Protein, CF
Biotinylated Recombinant Human Fc epsilon RI alpha Avi-tag His-tag Protein Binding Activity.
When Human IgE is immobilized at 1.00 µg/mL (100 µL/well), Biotinylated Recombinant Human Fc epsilon RI alpha Avi-tag His-tag Protein (Catalog # AVI6678) binds with an ED50 of 0.300 - 1.80 ng/mL.Biotinylated Recombinant Human Fc epsilon RI alpha Avi-tag His-tag Protein SDS-PAGE.
2 μg/lane of Biotinylated Recombinant Human Fc epsilon RI alpha Avi-tag His-tag Protein (Catalog # AVI6678) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 50-60 kDa.Formulation, Preparation and Storage
AVI6678
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. |
| Reconstitution | Reconstitute at 500 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: Fc epsilon RI alpha
The alpha subunit of the high affinity IgE receptor (Fc epsilon RI alpha or Fc epsilonRIA) is an IgE‑binding type I transmembrane glycoprotein of the multichain immune recognition (MIRR) family (1, 2). The receptor, Fc epsilon RI, is a tetrameric complex of one alpha, one beta and two gamma subunits ( alpha beta gamma2) on mast cells and basophils (1). An alternate trimeric form ( alpha gamma2) is expressed on human, but not rodent, mast cells, basophils, eosinophils and professional antigen presenting cells (3). While the gamma subunit is essential for expression of Fc epsilon RI alpha on the cell surface and for cell signaling, the beta subunit, when present, increases the halflife of the Fc epsilon RI complex on the cell surface (3, 4). An isoform of the beta subunit, betaT, blocks processing of the alpha subunit and its cell surface expression (2, 3, 5). Human Fc epsilon RI alpha cDNA encodes 257 amino acids (aa) including a 25 aa signal sequence, a 180 aa extracellular domain containing two Ig‑like domains that bind IgE and an endoplasmic reticulum retention motif, a 21 aa transmembrane domain with a charged amino acid (Asp219) that contributes to intracellular transport, and a 32 aa cytoplasmic sequence (1, 3, 6). Human Fc epsilon RI alpha shares 50-62% aa sequence identity with mouse, rat, equine, ovine, bovine, porcine and canine Fc epsilon RI alpha. Binding of IgE alone increases surface expression of Fc epsilon RI, while crosslinking of IgE/Fc epsilon RI complexes by IgE ligands (allergens) initiates receptor internalization and signaling (2, 4, 5). Mast cell and basophil activation by IgE/Fc epsilon RI crosslinking causes degranulation, releasing histamine, leukotrienes, prostaglandins, and other mediators of immediate‑type and late‑phase allergic reactions. Circulating autoantibodies that crosslink Fc epsilon RI alpha are often found in patients with chronic urticaria (7). Fc epsilon RI on human antigen presenting cells mediates uptake and processing of allergens for presentation by class II MHC (2, 3). Fc epsilon RI expression on human DC and Langerhans cells is up‑regulated during allergic reactions (atopy) and correlates with serum IgE concentration (3). Our Avi-tag Biotinylated Human Fc epsilon RI alpha protein features biotinylation at a single site contained within the Avi-tag, a unique 15 amino acid peptide. Protein orientation will be uniform when bound to streptavidin-coated surface due to the precise control of biotinylation and the rest of the protein is unchanged so there is no interference in the protein's bioactivity.
References
- Shimizu, A. et al. (1988) Proc. Natl. Acad. Sci. USA 85:1907.
- Abramson, J. and I. Pecht (2007) Immunol. Rev. 217:231.
- Kraft, S. and J-P. Kinet (2007) Nat. Rev. Immunol. 7:365.
- Yamasaki, S. and T. Saito (2008) J. Pharmacol. Sci. 106:336.
- Brenzovich, J. et al. (2009) J. Leukoc. Biol. 86:1351.
- Cauvi, D.M. et al. (2006) J. Biol. Chem. 281:10448.
- Kikuchi, Y. et al. (2001) J. Allergy Clin. Immunol. 107:1056.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional Fc epsilon RI alpha Products
Product Documents for Recombinant Human Fc epsilon RI alpha Avi His Protein, CF
Product Specific Notices for Recombinant Human Fc epsilon RI alpha Avi His Protein, CF
For research use only