Recombinant Human IL-15 Protein, Animal-Free Best Seller
R&D Systems, part of Bio-Techne | Catalog # BT-015-AFL

New! Bypass reconstitution steps by using a liquid formulation of Animal-free Recombinant Human IL-15. Find out more here.
Key Product Details
Product Specifications
Source
Asn49-Ser162
Produced using non-animal reagents in an animal-free laboratory.
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
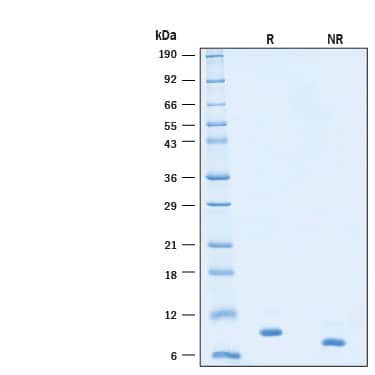
SDS-PAGE
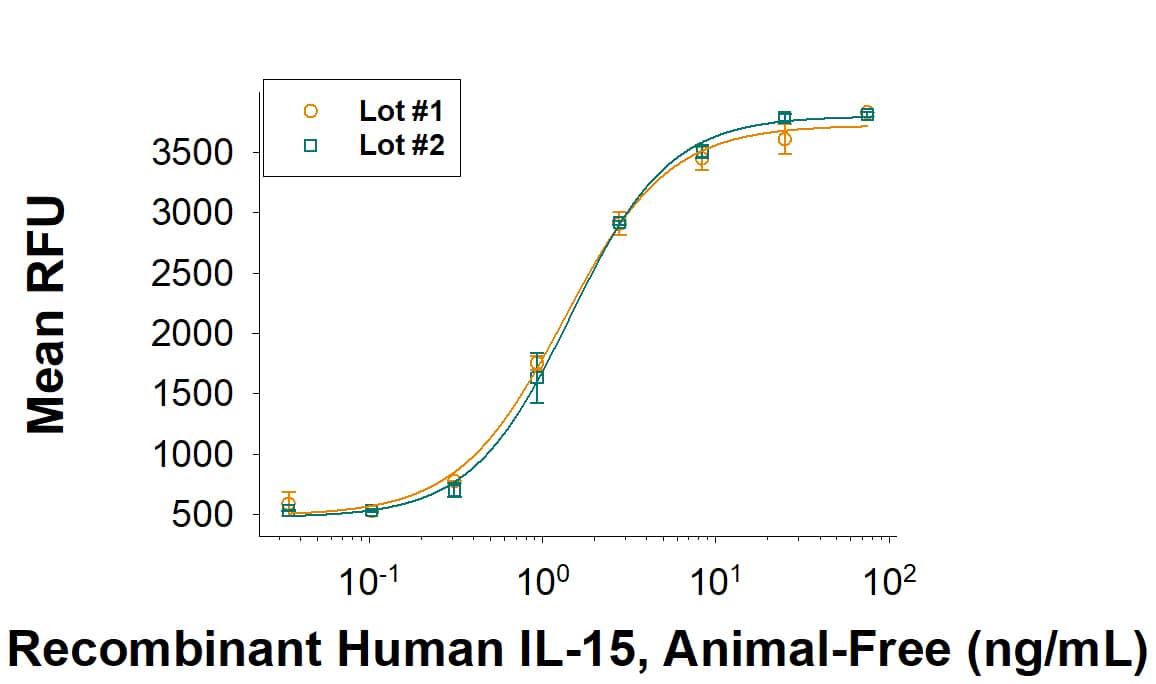
Activity
The ED50 for this effect is 0.300-2.60 ng/mL. The specific activity of recombinant human IL-15 is >2.00 x 108 units/mg, which is calibrated against the human IL-15 reference standard (NIBSC code: 95/554).
Scientific Data Images for Recombinant Human IL-15 Protein, Animal-Free
Recombinant Human IL-15 Protein, Animal-Free, Bioactivity.
Animal-FreeTM Recombinant Human IL-15 (Catalog # BT-015-AFL) stimulates cell proliferation in the MO7e human megakaryocytic leukemic cell line. The ED50for this effect is 0.300-2.60 ng/mL. Two independent lots were tested for activity and plotted on the same graph to show lot-to-lot consistency of Animal-Free IL-15.Recombinant Human IL-15 Protein, Animal-Free, SDS-PAGE.
2 μg/lane of Recombinant Human IL-15 Protein, Animal-Free (Catalog # BT-015-AFL) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 9 kDa.Formulation, Preparation and Storage
Lyophilized: BT-015-AFL
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. |
| Reconstitution | Reconstitute at 100-500 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Liquid: BT-015-AFL/LQ
| Formulation | Supplied as a 0.2 μm filtered solution in PBS. |
| Shipping | The product is shipped with dry ice or equivalent. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: IL-15
Interleukin 15 (IL-15) is a widely expressed 14 kDa cytokine that is structurally and functionally related to IL-2 and plays an important role in many immunological diseases (1, 2). Mature human IL-15 shares 70% amino acid sequence identity with mouse and rat IL-15. Alternative splicing generates isoforms of IL-15 with either a long or short signal peptide (LSP or SSP), and the SSP isoform is retained intracellularly (3). IL-15 binds with high affinity to IL-15 R alpha (4). It binds with lower affinity to a complex of IL-2 R beta and the common gamma chain ( gammac) which are also subunits of the IL-2 receptor complex (5). IL-15 associates with IL-15 R alpha in the endoplasmic reticulum, and this complex is expressed on the cell surface (6). The dominant mechanism of IL-15 action is known as transpresentation in which IL-15 and IL-15 R alpha are coordinately expressed on the surface of one cell and interact with complexes of IL-2 R beta/ gammac on adjacent cells (7). This enables cells to respond to IL-15 even if they do not express IL-15 R alpha (6).
In human and mouse, soluble IL-15-binding forms of IL-15 R alpha can be generated by proteolytic shedding and bind up nearly all the IL-15 in circulation (8-10). Soluble IL-15 R alpha functions as an inhibitor that limits IL-15 action (4, 9). Ligation of membrane-associated IL-15/IL-15 R alpha complexes also induces reverse signaling that promotes activation of the IL-15/IL-15 R alpha expressing cells (11). IL-15 induces or enhances the differentiation, maintenance, or activation of multiple T cell subsets including NK, NKT, Th17, Treg, and CD8+ memory cells (12 - 16). An important component of these functions is the ability of IL‑15 to induce dendritic cell differentiation and inflammatory activation (11, 14). IL-15 exhibits anti-tumor activity independent of its actions on NK cells or CD8+ T cells (17). It also inhibits the deposition of lipid in adipocytes, and its circulating levels are decreased in obesity (18).
Immunotherapy treatment with recombinant IL-15 has the advantage of not stimulating Treg cells like IL-2 does but has the drawback of associated toxicity at higher doses. This has led to increased investigation on mitigating IL-15 toxicity and combination immunotherapy approaches using immune checkpoint inhibitors (19, 20). Preclinical and early clinical studies have shown the potential of also using IL-15 in combination with cancer vaccines to improve their anti-tumor response (20). IL-15 can also be used for the preconditioning of CAR T cells or for engineering cells to express IL-15 in vivo. Adoptive cell transfer of NK cells engineered to express CD19 and IL-15 were well tolerated in patients with CD19-positive cancers (20).
IL-15 can be used in combination with other cytokines like IL-21 to increase the efficiency of NK cell expansion and maturation in stem cell culture protocols (21). The combination of IL-15 with IL-7 also promotes expansion of early-differentiated CD8+ T cells in culture with the added benefit of decreasing Treg cell generation, unlike IL-2, for adoptive cell transfer in cancer immunotherapy (22). GMP IL-7 and GMP IL-15 are commonly used in combination for ex vivo expansion of T cells for cellular therapies.
References
- De Sabatino, A. et al. (2011) Cytokine Growth Factor Rev. 22:19.
- Grabstein, K. et al. (1994) Science 264:965.
- Tagaya, Y. et al. (1997) Proc. Natl. Acad. Sci. USA 94:14444.
- Giri, J.G. et al. (1995) EMBO J. 14:3654.
- Giri, J. et al. (1994) EMBO J. 13:2822.
- Dubois, S. et al. (2002) Immunity 17:537.
- Castillo, E.F. and K.S. Schluns (2012) Cytokine 59:479.
- Budagian, V. et al. (2004) J. Biol. Chem. 279:40368.
- Mortier, E. et al. (2004) J. Immunol. 173:1681.
- Bergamaschi, C. et al. (2012) Blood 120:e1.
- Budagian, V. et al. (2004) J. Biol. Chem. 279:42192.
- Mortier, E. et al. (2003) J. Exp. Med. 205:1213.
- Gordy, L.E. et al. (2011) J. Immunol. 187:6335.
- Harris, K.M. (2011) J. Leukoc. Biol. 90:727.
- Xia, J. et al. (2010) Clin. Immunol. 134:130.
- Schluns, K.S. et al. (2002) J. Immunol. 168:4827.
- Davies, E. et al. (2010) J. Leukoc. Biol. 88:529.
- Barra, N.G. et al. (2010) Obesity 18:1601.
- Xue, D. et al. (2021) Antib Ther. 4:123.
- Wolfarth, A.A. et al. (2022) Immune Netw. 22:e5.
- Oberoi, P. et al. (2020). Cells. 9:811.
- Chamucero-Millares, J.A. et al. (2021) Cellular Immunol. 360:104257.
Long Name
Alternate Names
Entrez Gene IDs
Gene Symbol
UniProt
Additional IL-15 Products
Product Documents for Recombinant Human IL-15 Protein, Animal-Free
Manufacturing Specifications
Animal-Free Manufacturing ConditionsOur dedicated controlled-access animal-free laboratories ensure that at no point in production are the products exposed to potential contamination by animal components or byproducts. Every stage of manufacturing is conducted in compliance with R&D Systems' stringent Standard Operating Procedures (SOPs). Production and purification procedures use equipment and media that are confirmed animal-free.
Production
- All molecular biology procedures use animal-free media and dedicated labware.
- Dedicated fermentors are utilized in committed animal-free areas.
Purification
- Protein purification columns are animal-free.
- Bulk proteins are filtered using animal-free filters.
- Purified proteins are stored in animal-free containers in a dedicated cold storage room.
Quality Assurance
- Low Endotoxin Level.
- No impairment of biological activity.
- High quality product obtained under stringent conditions.
- For ex vivo research or bioproduction, additional documentation can be provided.
Product Specific Notices for Recombinant Human IL-15 Protein, Animal-Free
For research use only