Recombinant Human IL-23R Fc Chimera Avi-tag Protein, CF
R&D Systems, part of Bio-Techne | Catalog # AVI1400

Key Product Details
Source
Structure / Form
Biotinylated via Avi-tag
Conjugate
Applications
Product Specifications
Source
| Human IL-23R (Gly24-Asp353) Accession # AAM44229.1 |
IEGRMD | Human IgG1 (Pro100-Lys330) |
Avi-tag |
| N-terminus | C-terminus | ||
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
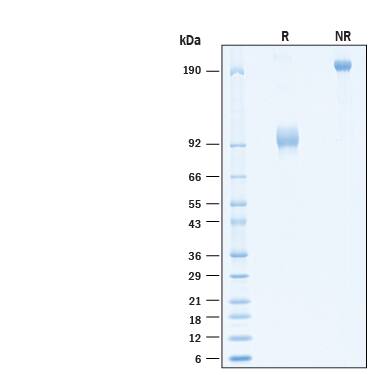
SDS-PAGE
Activity
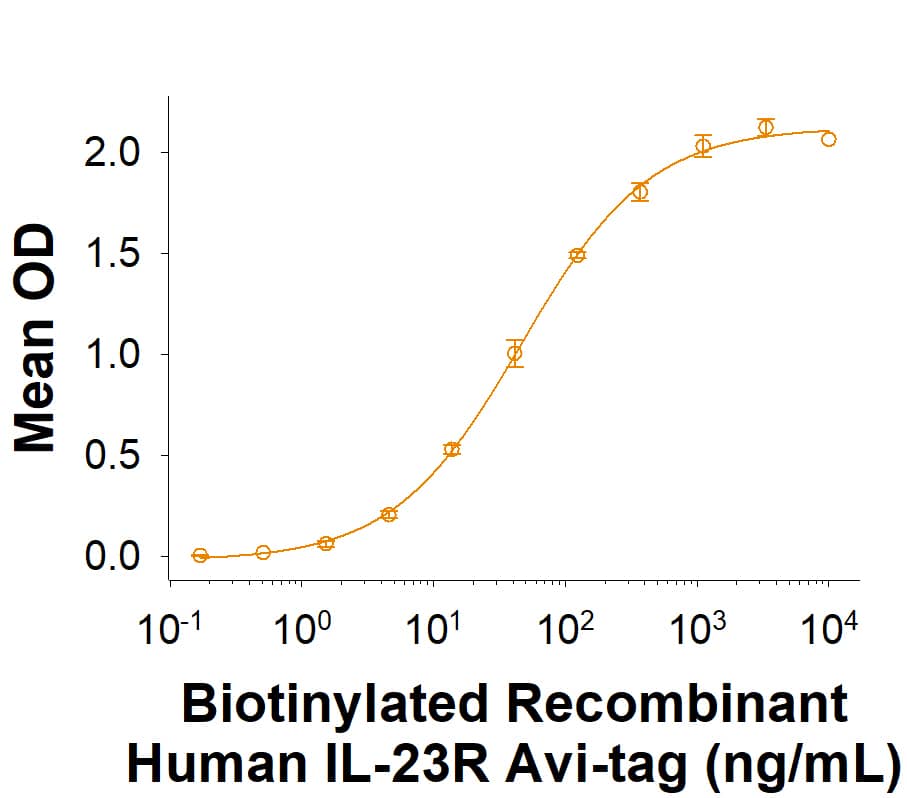
When Recombinant Human IL-23 Protein (Catalog # 1290-IL) is immobilized at 1.0 µg/mL (100 µL/well), the concentration of Biotinylated Recombinant Human IL-23R Fc Chimera Avi-tag (Catalog # AVI1400) that produces 50% of the optimal binding response is 10.0-100 ng/mL.
Scientific Data Images for Recombinant Human IL-23R Fc Chimera Avi-tag Protein, CF
Biotinylated Recombinant Human IL-23R Fc Chimera Avi-tag Protein Binding Activity
When Recombinant Human IL-23 Protein (1290-IL) is immobilized at 1.0 µg/mL (100 µL/well), the concentration of Biotinylated Recombinant Human IL-23R Fc Chimera Avi-tag Protein (Catalog # AVI1400) that produces 50% of the optimal binding response is 10.0-100 ng/mL.Biotinylated Recombinant Human IL-23R Fc Chimera Avi-tag Protein SDS-PAGE.
2 μg/lane of Biotinylated Recombinant Human IL-23R Fc Chimera Avi-tag Protein (Catalog # AVI1400) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 90-102 kDa and 180-200 kDa, respectively.Formulation, Preparation and Storage
AVI1400
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. |
| Reconstitution | Reconstitute at 250 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: IL-23R
Interleukin 23 (IL-23) is a heterodimeric cytokine composed of two disulfide-linked subunits, a p19 subunit that is unique to IL-23, and a p40 subunit that is shared with IL-12 (1 - 5). The functional IL-23 receptor complex consists of two receptor subunits, the IL-12 receptor beta 1 subunit (IL-12 R beta1) and the IL-23-specific receptor subunit (IL-23 R) (3). Human IL-23 R cDNA encodes a 629 aa type I transmembrane protein with a 23 aa residue signal peptide, a 330 aa residue extracellular domain, a 23 aa residue transmembrane domain and a 253 aa residue cytoplasmic region. IL-23 R shares structural features with the IL-12 R beta2, including an N-terminal Ig-like domain, two cytokine receptor domains and multiple glycosylation sites in the extracellular domain. IL-23 R lacks the three extracellular membrane-proximal fibronectin-type III domains present on IL-12 R beta2. IL-23 R has a WQPWS sequence in the transmembrane-proximal cytokine receptor domain similar to the cytokine receptor signature WSXWS motif. The cytoplasmic region of IL-23 R has three potential Src homology 2 domain-binding sites and two potential Stat-binding sites. The gene for human IL-23 R is located on human chromosome 1 within 150 kb of IL-12 R beta2. Human and mouse IL-23 R share 66% amino acid sequence identity. Based on quantitative real-time PCR, human IL-23 R mRNA is expressed in a human Th1 and Th0 clone as well as several NK cell lines and clones. Low but detectable levels of IL-23 R mRNA is also expressed in EBV-transformed B cells and activated PBMC. IL-23 initiates a signal transduction cascade similar to that of IL-12, and involves Jak2, Tyk2, Stat1, Stat3, Stat4, and Stat5. IL-23 has biological activities that are similar to, but distinct from IL-12. Our Avi-tag Biotinylated human IL-23R Fc chimera features biotinylation at a single site contained within the Avi-tag, a unique 15 amino acid peptide. Protein orientation will be uniform when bound to streptavidin-coated surface due to the precise control of biotinylation and the rest of the protein is unchanged so there is no interference in the protein's bioactivity.
References
- Oppmann, B. et al. (2000) Immunity 13:715.
- Lankford, C.S. and D.M. Frucht (2003) J. Leukoc. Biol. 73:49.
- Parham, C. et al. (2002) J. Immunol. 168:5448.
- Belladonna, M.L. et al. (2002) J. Immunol. 168:5448.
- Aggarwal, S. et al. (2003) J. Biol. Chem. 278:1910.
Long Name
Alternate Names
Gene Symbol
Additional IL-23R Products
Product Documents for Recombinant Human IL-23R Fc Chimera Avi-tag Protein, CF
Product Specific Notices for Recombinant Human IL-23R Fc Chimera Avi-tag Protein, CF
For research use only