Recombinant Human IL-5 R alpha/CD125 Fc Avi-tag Protein, CF
R&D Systems, part of Bio-Techne | Catalog # AVI11073

Key Product Details
Source
Accession #
Structure / Form
Biotinylated via Avi-tag
Conjugate
Applications
Product Specifications
Source
| Human IL-5RA (Asp21-Glu335) Accession # Q01344.2 |
IEGRMD | Human IgG1 Fc (Pro100-Lys330) |
Avi-tag |
| N-terminus | C-terminus | ||
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
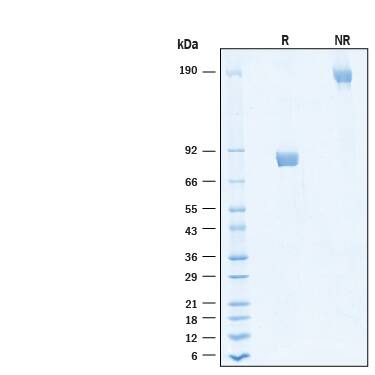
SDS-PAGE
Activity
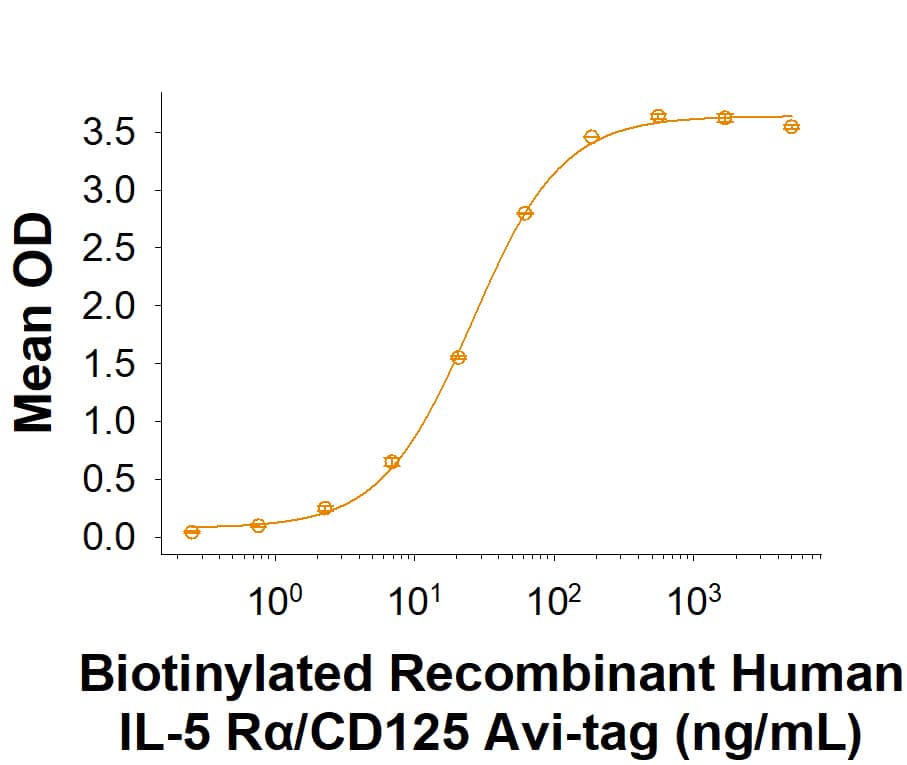
When Recombinant Human IL-5 (Catalog # 205-IL/CF) is immobilized at 0.5 µg/mL (100 µL/well), Biotinylated Recombinant Human IL-5 R alpha/CD125 Fc Chimera Avi-tag (Catalog # AVI11073) binds with an ED50 of 6.00-90.0 ng/mL.
Scientific Data Images for Recombinant Human IL-5 R alpha/CD125 Fc Avi-tag Protein, CF
Biotinylated Recombinant Human IL‑5 R alpha/CD125 Fc Chimera Avi-tag Protein Binding Activity
When Recombinant Human IL-5 (205-IL/CF) is immobilized at 0.5 µg/mL (100 µL/well), Biotinylated Recombinant Human IL-5 R alpha/CD125 Fc Chimera Avi-tag Protein (Catalog # AVI11073) binds with an ED50 of 6.00-90.0 ng/mL.Biotinylated Recombinant Human IL‑5 R alpha/CD125 Fc Chimera Avi-tag Protein SDS-PAGE
2 μg/lane of Biotinylated Recombinant Human IL‑5 R alpha/CD125 Fc Chimera Avi-tag Protein (Catalog # AVI11073) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 78-87 kDa and 160-170 kDa, respectively.Formulation, Preparation and Storage
AVI11073
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. |
| Reconstitution | Reconstitute at 500 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: IL-5 R alpha/CD125
Interleukin‑5 Receptor alpha (IL‑5 R alpha), also known as CD125, is a 60 kDa hematopoietin receptor that plays a dominant role in eosinophil biology (1‑3). Mature human IL‑5 R alpha consists of a 322 amino acid (aa) extracellular domain (ECD) with a WSxWS motif and a four cysteine motif, a 20 aa transmembrane segment, and a 58 aa cytoplasmic domain (4, 5). Within the ECD, human IL-5 R alpha shares 71% aa sequence identity with mouse and rat IL‑5 R alpha. Alternate splicing of human IL‑5 R alpha generates soluble secreted forms which function as IL‑5 antagonists (5‑7). The high affinity receptor for IL‑5 is a complex that consists of the ligand binding IL‑5 R alpha and the transmembrane common beta chain ( betac/CD131) which is shared with the receptor complexes for IL‑3 and GM‑CSF (4). IL‑5 R alpha binds IL‑5 at low affinity and then associates with preformed betac oligomers to form the signaling‑competent receptor complex (8). IL‑5 stimulation of CD34+ hematopoietic progenitor cells induces the up‑regulation of transmembrane IL‑5 R alpha followed by eosinophilic differentiation and activation (9 ‑ 11). IL‑5 R alpha also promotes the differentiation of basophils and B cells (12, 13). Exposure of mature eosinophils to IL‑5 attenuates their IL‑5 responsiveness by inducing the down‑regulation of surface IL‑5 R alpha and increased production of soluble IL‑5 R alpha (14, 15). Elevated production of IL‑5 at sites of allergic inflammation induces eosinophilia and exacerbation of immune cell infiltration, tissue damage, and remodeling (2, 3). Our Avi-tag Biotinylated human IL-5 R alpha Fc chimera features biotinylation at a single site contained within the Avi-tag, a unique 15 amino acid peptide. Protein orientation will be uniform when bound to streptavidin-coated surface due to the precise control of biotinylation and the rest of the protein is unchanged so there is no interference in the protein's bioactivity.
References
- Martinez-Moczygemba, M. and D.P. Huston (2003) J. Allergy Clin. Immunol. 112:653.
- Rothenberg, M.E. and S.P. Hogan (2005) Annu. Rev. Immunol. 24:147.
- Elsas, X.P. and M.I.G. Elsas (2007) Curr. Med. Chem. 14:1925.
- Tavernier, J. et al. (1991) Cell 66:1175.
- Murata, Y. et al. (1992) J. Exp. Med. 175:341.
- Tavernier, J. et al. (1992) Proc. Natl. Acad. Sci. 89:7041.
- Cameron, L. et al. (2000) J. Immunol. 164:1538.
- Zaks-Zilberman, M. et al. (2008) J. Biol. Chem. 283:13398.
- Tavernier, J. et al. (2000) Blood 95:1600.
- Clutterbuck, E.J. et al. (1989) Blood 73:1504.
- Lopez, A.F. et al. (1988) J. Exp. Med. 167:219.
- Denburg, J.A. et al. (1991) Blood 77:1462.
- Hasbold, J. et al. (2004) Nat. Immunol. 5:55.
- Gregory, B. et al. (2003) J. Immunol. 170:5359.
- Liu, L.Y. et al. (2002) J. Immunol. 169:6459.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional IL-5 R alpha/CD125 Products
Product Documents for Recombinant Human IL-5 R alpha/CD125 Fc Avi-tag Protein, CF
Product Specific Notices for Recombinant Human IL-5 R alpha/CD125 Fc Avi-tag Protein, CF
For research use only