Recombinant Human KGF/FGF-7 GMP Protein, CF GMP Best Seller
R&D Systems, part of Bio-Techne | Catalog # 251-GMP

Key Product Details
Product Specifications
Source
Cys32-Thr194, with an N-terminal Met
Manufactured and tested under cGMP guidelines.
Purity
Endotoxin Level
N-terminal Sequence Analysis
(Cys)32-Asn-Asp-Met-Thr-Pro-Glu-Gln-Met-Ala
Predicted Molecular Mass
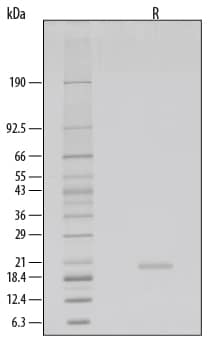
SDS-PAGE
Activity
The ED50 for this effect is 6-60 ng/mL.
The specific activity of recombinant human KGF/FGF-7 is >8.16 x 105 units/mg, which is calibrated against the human KGF/FGF-7 WHO Standard (NIBSC code: 03/150).
Host Cell Protein
Mycoplasma
Host Cell DNA
Scientific Data Images for Recombinant Human KGF/FGF-7 GMP Protein, CF
Recombinant Human KGF/FGF-7 GMP Protein Bioactivity
GMP-grade Recombinant Human KGF/FGF-7 (Catalog # 251-GMP) stimulates pro-liferation of the 4MBr‑5 rhesus monkey epithelial cell line. The ED50 for this effect is 6-60 ng/mL.Recombinant Human KGF/FGF-7 GMP Protein SDS-PAGE
1 μg/lane of GMP-grade Recombinant Human KGF/FGF-7 (Catalog # 251-GMP) was resolved with SDS-PAGE under reducing (R) conditions and visualized by silver staining, showing a single band at 20 kDa.Formulation, Preparation and Storage
251-GMP
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS. |
| Reconstitution | Reconstitute at 100 μg/mL in PBS. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: KGF/FGF-7
KGF (keratinocyte growth factor), also known as FGF-7 (fibroblast growth factor-7), is one of 22 known members of the mouse FGF family of secreted proteins that plays a key role in development, morphogenesis, angiogenesis, wound healing, and tumorigenesis (1-4). KGF expression is restricted to cells of mesenchymal origin. When secreted, it acts as a paracrine growth factor for nearby epithelial cells (1). KGF speeds wound healing by being dramatically upregulated in response to damage to skin or internal structures that results in high local concentrations of inflammatory mediators such as IL-1 and TNF-alpha. (2, 5). KGF promotes cell migration and invasion, and mediates melanocyte transfer to keratinocytes upon UVB radiation (6, 7). It has been used ectopically to avoid chemotherapy-induced oral mucositis in patients with hematological malignancies (1). Deletion of KGF affects kidney development, producing abnormally small ureteric buds and fewer nephrons (8). It also impedes hair follicle differentiation (9). The 194 amino acid (aa) KGF precursor contains a 31 aa signal sequence and, like all other FGFs, an ~120 aa beta-trefoil scaffold that includes receptor- and heparin-binding sites. KGF signals only through the IIIb splice form of the tyrosine kinase receptor, FGF R2 (FGF R2-IIIb/KGF R) (10). Receptor dimerization requires an octameric or larger heparin or heparin sulfate proteoglycan (11). FGF-10, also called KGF2, shares 51% aa identity and similar function to KGF, but shows more limited expression than KGF and uses an additional receptor, FGF R2-IIIc (12). Following receptor engagement, KGF is typically degraded, while FGF-10 is recycled (12). Mature human KGF, which is active across species, shares 98% aa sequence identity with bovine, equine, ovine and canine, 96% with mouse and porcine, and 92% with rat KGF, respectively.
References
- Finch, P.W. and J.S. Rubin (2006) J. Natl. Cancer Inst. 98:812.
- Werner, S. et al. (2007) J. Invest. Dermatol. 127:998.
- Werner, S. (1998) Cytokine Growth Factor Rev. 9:153.
- Mason, I.J. et al. (1994) Mech. Dev. 45:15.
- Geer, D.J. et al. (2005) Am. J. Pathol. 167:1575.
- Niu, J. et al. (2007) J. Biol. Chem. 282:6001.
- Cardinali, G. et al. (2005) J. Invest. Dermatol. 125:1190.
- Qiao, J. et al. (1999) Development 126:547.
- Guo, L. et al. (1996) Genes Dev. 10:165.
- de Georgi, V. et al. (2007) Dermatol. Clin. 25:477.
- Hsu, Y-R. et al. (1999) Biochemistry 38:2523.
- Belleudi, F. et al. (2007) Traffic 8:1854.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional KGF/FGF-7 Products
Product Documents for Recombinant Human KGF/FGF-7 GMP Protein, CF
Manufacturing Specifications
GMP ProteinsR&D Systems, a Bio-Techne Brand's GMP proteins are produced according to relevant sections of the following documents: USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Eu. Ph. 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products.
R&D Systems' quality focus includes:
- Manufactured and tested under an ISO 9001:2015 and ISO 13485:2016 certified quality system
- Documented processes and QA control of documentation and process changes
- Personnel training programs
- Raw material testing and vendor qualification/monitoring
- Fully validated equipment, processes and test methods
- Equipment calibration schedules using a computerized calibration program
- Facility maintenance, safety programs and pest control
- Material review process for variances
- Monitoring of stability over product shelf-life
- N-terminal amino acid analysis, SDS-PAGE analysis, and endotoxin level (as determined by LAL assay) performed on each bulk QC lot, not on individual bottlings of each QC lot
- Post-bottling lot-specific bioassay results (compliance with an established range) and results of microbial testing according to USP
- Host Cell Protein testing performed by ELISA
- Mycoplasma testing by ribosomal RNA hybridization assay
Production records and facilities are available for examination by appropriate personnel on-site at R&D Systems in Minneapolis, Minnesota USA.
R&D Systems sells GMP grade products for preclinical or clinical ex vivo use. They are not for in vivo use. Please read the following End User Terms prior to using this product.
Product Specific Notices for Recombinant Human KGF/FGF-7 GMP Protein, CF
END USER TERMS OF USE OF PRODUCT
The following terms are offered to you upon your acceptance of these End User Terms of Use of Product. By using this product, you indicate your acknowledgment and agreement to these End User Terms of Use of Product. If you do not agree to be bound by and comply with all of the provisions of these End User Terms of Use of Product, you should contact your supplier of the product and make arrangements to return the product.
We suggest you print and retain a copy of these End User Terms of Use of Product for your records.
The End User is aware that R&D Systems, Inc. sells GMP products for preclinical or clinical ex vivo use and not for in vivo use. The End User further agrees, as a condition of the sale of R&D Systems' GMP products that: a) the End User will not use this GMP Product in any procedure wherein the product may be directly or indirectly administered to humans, unless the End User has obtained, or prior to their use will have obtained, an Investigational New Drug (IND) exemption from the FDA and will use the product only in accordance with the protocols of such IND and of the Institutional Review Board overseeing the proposed research, or b) the End User will use the products outside of the United States in accordance with the protocols of research approved by the Institutional Review Board or authorized ethics committee and regulatory agencies to which the End User is subject to in their territory.
R&D Systems, Inc. has the right, at its sole discretion, to modify, add or remove any terms or conditions of these End User Terms of Use without notice or liability to you. Any changes to these End User Terms of Use are effective immediately following the printing of such changes on this product insert. The most recent version of these End User Terms of Use of Product may be found at: RnDSystems.com/Legal.
You agree to review these End User Terms of Use of Product to ensure any subsequent use by you of R&D Systems' GMP Products following changes to these End User Terms of Use of Product constitutes your acceptance of all such changes.
TERMS AND CONDITIONS
The following limitation applies to R&D Systems' warranty and liability for damages: All products are warranted to meet R&D Systems' published specifications when used under normal laboratory conditions.
R&D SYSTEMS DOES NOT MAKE ANY OTHER WARRANTY OR REPRESENTATION WHATSOEVER, WHETHER EXPRESS OR IMPLIED, WITH RESPECT TO ITS PRODUCTS. IN PARTICULAR, R&D SYSTEMS DOES NOT MAKE ANY WARRANTY OF SUITABILITY, NONINFRINGEMENT, MERCHANTABILITY OR FITNESS FOR ANY PARTICULAR PURPOSE.
NOTWITHSTANDING ANY OTHER PROVISIONS OF THESE TERMS AND/OR ANY OTHER AGREEMENT BETWEEN R&D SYSTEMS AND PURCHASER FOR THE PURCHASE OF THE PRODUCTS, R&D SYSTEMS' TOTAL LIABILITY TO PURCHASER ARISING FROM OR IN RELATION TO THESE TERMS, AN AGREEMENT BETWEEN THE PARTIES OR THE PRODUCTS, WHETHER ARISING IN CONTRACT, TORT OR OTHERWISE SHALL BE LIMITED TO THE TOTAL AMOUNT PAID BY PURCHASER TO R&D SYSTEMS FOR THE APPLICABLE PRODUCTS. IN NO EVENT WILL R&D SYSTEMS BE LIABLE FOR THE COST OF PROCUREMENT OF SUBSTITUTE GOODS.
Full details of R&D Systems' Terms and Conditions of Sale can be found online at: RnDSystems.com/Legal.
For preclinical, or clinical ex vivo use