Recombinant Human PDGF R alpha Fc Chimera Avi Protein, CF
R&D Systems, part of Bio-Techne | Catalog # AVI6765

Key Product Details
Source
Accession #
Structure / Form
Conjugate
Applications
Product Specifications
Source
| Human PDGFRA (Gln24-Glu524) Accession # P16234.1 |
IEGRMD | Human IgG1 (Pro100-Lys330) |
Avi-tag |
| N-terminus | C-terminus | ||
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
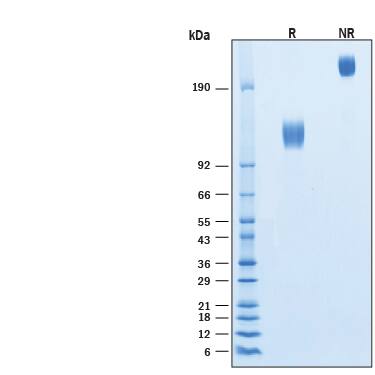
SDS-PAGE
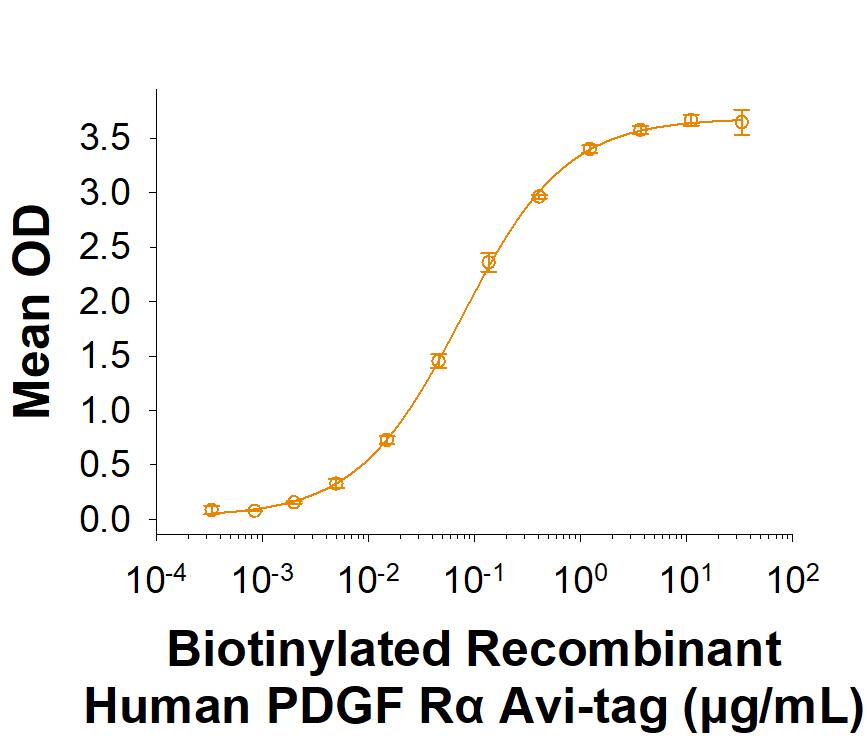
Activity
When Recombinant Human PDGF-AA (Catalog # 221-AA) is immobilized at 0.5 µg/mL (100 µL/well), Biotinylated Recombinant Human PDGF R alpha Fc Chimera Avi-tag (Catalog # AVI6765) binds with an ED50 of 0.0400-0.480 µg/mL.
Scientific Data Images for Recombinant Human PDGF R alpha Fc Chimera Avi Protein, CF
Biotinylated Recombinant Human PDGF R alpha Fc Chimera Avi-tag Protein Binding Activity.
When Recombinant Human PDGF‑AA (221-AA) is immobilized at 0.5 µg/mL (100 µL/well), Biotinylated Recombinant Human PDGF R alpha Fc Chimera Avi-tag Protein (Catalog # AVI6765) binds with an ED50 of 0.0400-0.480 µg/mL.Biotinylated Recombinant Human PDGF R alpha Fc Chimera Avi-tag Protein SDS-PAGE.
2 μg/lane of Biotinylated Recombinant Human PDGF R alpha Fc Chimera Avi-tag Protein (Catalog # AVI6765) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 115-130 kDa and 230-260 kDa, respectively.Formulation, Preparation and Storage
AVI6765
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. |
| Reconstitution | Reconstitute at 500 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: PDGF R alpha
PDGF R alpha (platelet-derived growth factor receptor alpha) is a type I transmembrane glycoprotein in the class III subfamily of receptor tyrosine kinases (RTK) (1 ‑ 4). PDGF R alpha and PDGF R beta can form homo- or hetero-dimeric receptors when engaged by dimers of the PDGF family of growth factors, which include disulfide-linked homodimers of PDGF-A, B, C or D, or the heterodimer PDGF-AB that is mainly found in human platelets. While multiple in vitro ligand-receptor combinations have been identified, in vivo evidence indicates that PDGF R alpha primarily binds PDGF-AA and PDGF-CC, while PDGF R beta primarily binds PDGF-BB and probably PDGF-DD. Like all class III RTKs, the extracellular domain (ECD) of human PDGF R alpha (aa 24 ‑ 524) contains five immunoglobulin-like domains, while the intracellular region contains a split tyrosine kinase domain (aa 593 ‑ 954) (1 ‑ 4). Within the ECD, human PDGF R alpha shares 85%, 83%, 95%, 93%, and 88% aa sequence identity with mouse, rat, equine, canine and bovine PDGF R alpha respectively. PDGF R alpha autophosphorylates upon dimerization, activating signaling cascades in PI 3-kinase Ras‑MAP kinase, and PLC-gamma pathways (1, 2). Signaling is down‑regulated by SHP-2 phosphatase activity and by receptor endocytosis and lysosomal degradation. PDGF R alpha is expressed at low levels in most mesenchymal cells, but is strongly expressed in oligodendrocyte, lung, skin and intestinal progenitor cells and induced by inflammation or growth in culture (1 ‑ 4). During development, mesenchymal cells expressing PDGF R alpha respond to local gradients of epithelially produced PDGF-AA or PDGF-CC during formation of the cranial and cardiac neural crest, retina, gonads, lung alveoli, intestinal villi, skin, hair follicles, skeleton, teeth, palate, and interstitial kidney mesenchyme (1, 5). Deletion of PDGF R alpha in mice severely impairs mesenchymal derivatives in both embryo and extraembryonic tissues, and high or low PDGF R alpha signaling in humans may result in spina bifida or cleft palate‑type malformations. Postnatally, PDGF R alpha is implicated in gliomas and fibrotic disorders of lung, heart and skin (scleroderma) (6, 8). Our Avi-tag Biotinylated PDGF R alpha Fc Chimeria features biotinylation at a single site contained within the Avi-tag, a unique 15 amino acid peptide. Protein orientation will be uniform when bound to streptavidin-coated surface due to the precise control of biotinylation and the rest of the protein is unchanged so there is no interference in the protein's bioactivity.
References
- Andrae, J. et al. (2008) Genes Dev. 22:1276.
- Heldin, C-H. and B. Westermark (1999) Physiol. Rev. 79:1283.
- Claesson-Welsh, L. et al. (1989) Proc. Natl. Acad. Sci. USA 86:4917.
- Matsui, T. et al. (1989) Science 243:800.
- Klinghoffer, R.A. et al. (2002) Dev. Cell 2:103.
- Martinho, O. (2009) Br. J. Cancer 101:973.
- Olson, L.E. and P. Soriano (2009) Dev. Cell 16:303.
- Baroni, S.S. et al. (2006) N. Engl. J. Med. 354:2667.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional PDGF R alpha Products
Product Documents for Recombinant Human PDGF R alpha Fc Chimera Avi Protein, CF
Product Specific Notices for Recombinant Human PDGF R alpha Fc Chimera Avi Protein, CF
For research use only