Recombinant SARS-CoV-2 BA.2 Spike RBD Fc Chimera Protein, CF
R&D Systems, part of Bio-Techne | Catalog # 11148-CV
Omicron Variant

Key Product Details
Product Specifications
Source
Human embryonic kidney cell, HEK293-derived sars-cov-2 Spike RBD protein
| SARS-CoV-2 BA.2 Spike RBD (Arg319-Phe541) (Gly339Asp, Ser371Phe, Ser373Pro, Ser375Phe, Thr376Ala, Asp405Asn, Arg408Ser, Lys417Asn, Asn440Lys, Ser477Asn, Thr478Lys, Glu484Ala, Gln493Arg, Gln498Arg, Asn501Tyr, Tyr505His) Accession # YP_009724390.1 |
IEGRMD | Human IgG1 (Pro100-Lys330) |
| N-terminus | C-terminus |
Purity
>95%, by SDS-PAGE visualized with Silver Staining and quantitative densitometry by Coomassie® Blue Staining.
Endotoxin Level
<0.10 EU per 1 μg of the protein by the LAL method.
N-terminal Sequence Analysis
Arg319
Predicted Molecular Mass
52 kDa
SDS-PAGE
59-65 kDa, under reducing conditions.
Activity
Measured by its binding ability in a functional ELISA with Recombinant
Human ACE-2 His-tag
(Catalog #
933-ZN).
Scientific Data Images for Recombinant SARS-CoV-2 BA.2 Spike RBD Fc Chimera Protein, CF
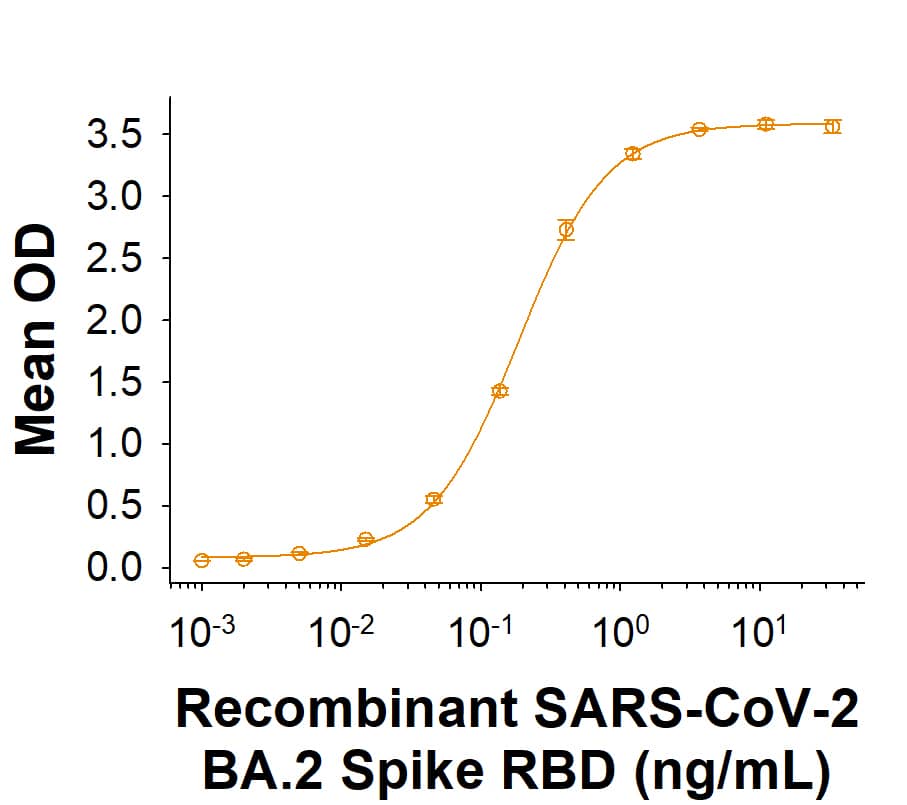
Recombinant SARS-CoV-2 BA.2 Spike RBD Fc Chimera Protein Binding Activity.
Recombinant SARS-CoV-2 BA.2 Spike RBD Fc Chimera Protein (Catalog # 11148-CV) binds Recombinant Human ACE-2 His-tag (933-ZN) in a functional ELISA.Recombinant SARS-CoV-2 BA.2 Spike RBD Fc Chimera Protein SDS-PAGE.
2 μg/lane of Recombinant SARS-CoV-2 BA.2 Spike RBD Fc Chimera Protein (Catalog # 11148-CV) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 59-65 kDa.Formulation, Preparation and Storage
11148-CV
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. |
| Reconstitution | Reconstitute at 500 μg/mL in PBS. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: Spike RBD
References
- Wu, F. et al. (2020) Nature 579:265.
- Tortorici, M.A. and D. Veesler (2019) Adv. Virus Res. 105:93.
- Bosch, B.J. et al. (2003) J. Virol. 77:8801.
- Belouzard, S. et al. (2009) Proc. Natl. Acad. Sci. 106:5871.
- Millet, J.K. and G.R. Whittaker (2015) Virus Res. 202:120.
- Li, W. et al. (2003) Nature 426:450.
- Wong, S.K. et al. (2004) J. Biol. Chem. 279:3197.
- Jiang, S. et al. (2020) Trends. Immunol. https://doi.org/10.1016/j.it.2020.03.007.
- Ortega, J.T. et al. (2020) EXCLI J. 19:410.
- Wrapp, D. et al. (2020) Science 367:1260.
- Tai, W. et al. (2020) Cell. Mol. Immunol. 17:613.
- Okba, N.M.A. et al. (2020). Emerg. Infect. Dis. https://doi.org/10.3201/eid2607.200841.
- Shah, M. and Woo, H.G. (2021) bioRxiv https://doi.org/10.1101/2021.12.04.471200.
- Lupala, C.S. et al. (2021) bioRxiv https://doi.org/10.1101/2021.12.10.472102.
Long Name
Spike Receptor Binding Domain
Gene Symbol
S
UniProt
Additional Spike RBD Products
Product Documents for Recombinant SARS-CoV-2 BA.2 Spike RBD Fc Chimera Protein, CF
Product Specific Notices for Recombinant SARS-CoV-2 BA.2 Spike RBD Fc Chimera Protein, CF
For research use only
Loading...
Loading...
Loading...