T Cell Exhaustion Phenotype Flow Cytometry Panel
Expression of inhibitory receptors is critical for control of an immune response. Aberrant and prolonged inhibitory receptor expression leads to immune dysfunction. Use this validated multicolor flow cytometry panel to characterize your T cells for exhaustion markers like PD-1, TIM-3 and Lag-3.
Flow Cytometry Panel for Immunophenotyping of Exhausted T Cells
| Marker | Clone | Fluorochrome | Catalog # |
| CD3 | UCHT1* | mFluor™ Violet 450 | FAB100MFV450 |
| CD4 | 11830 | mFluor™ Violet 500 | FAB3791MFV500 |
| CD8 | 37006 | Alexa Fluor® 700 | FAB1509N |
| Live/Dead | (APC-Cy7) | ||
| LAG-3 | 874501 | mFluor™ Violet 610 | FAB23193MFV610 |
| TIM-3 | 344823 | PE | FAB2365R |
| PD-1 | 2335a | Alexa Fluor® 647 | FAB10863R |
*Designate clones independently validated by HLDA.
Alexa Fluor® is registered trademark of Molecular Probes, Inc.
mFluor is a trademark of AAT Bioquest.
This multicolor flow cytometry panel was validated on human peripheral blood mononuclear cells (PBMCs).
Flow Cytometry Gating Strategy for T Cell Exhaustion Panels
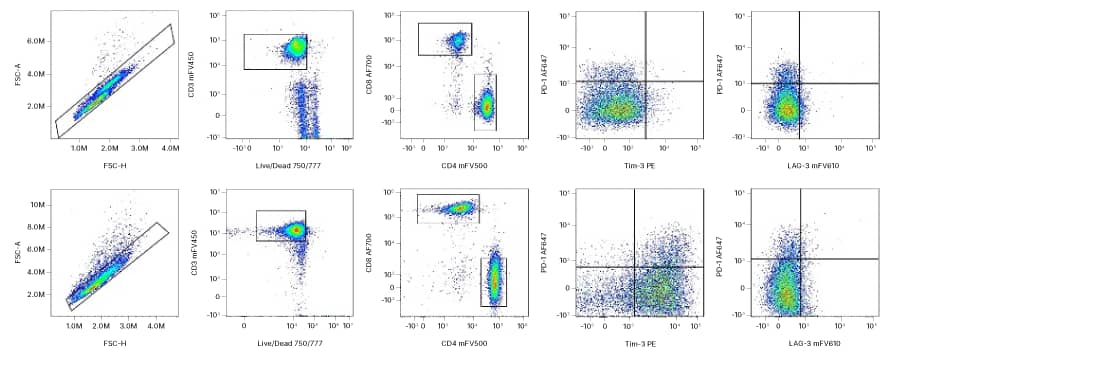
Multicolor flow cytometry panel to identify human Exhaustion T cell subsets. PBMCs stimulated with ahCD3 (1 ug/mL; Cat# MAB11411-GMP) and ahCD28 (3 ug/mL; Cat# MAB11412-GMP) coupled to M-270 Dynabeads + IL-2 (200 U/mL; Cat# 202-GMP) for 14 days. Naïve T cells and Day 9 expanded T cells were stained with anti-human CD3 mFluor™ Violet 450, CD4 mFluor™ Violet 500, CD8 Alexa Fluor® 700, PD-1 Alexa Fluor® 647, TIM-3 PE, and LAG-3 mFluor™ Violet 610. Gating strategy: Single Cells/Viable CD3+ cells/CD4+ vs. CD8+ cells. PD-1, LAG-3, and TIM-3 expression were examined on Viable CD3+ cells at Day 0 and Day 9 to determine T cell exhaustion.
Staining Protocol For T Cell Exhaustion Panel
Other supplies required
- PBS
- Flow Cytometry Staining Buffer (Catalog # FC001)
- Fc-block (blocking IgG)
- (Optional) Isotype Control Antibodies
- 5 mL Flow cytometry tubes
1. Wash human PBMCs (1 x 106 cells per sample) with 2 mL of Staining Buffer (1X) (Catalog # FC001) or other BSA-containing buffer, by spinning at 300 x g for 5 minutes, using 5 mL flow cytometry tubes. Decant/aspirate supernatant.
2. Fc-block cells with blocking IgG (1 μg IgG/106 cells) for 10 minutes at room temperature.
3. Add previously titrated amount of each primary conjugated antibody. Vortex tubes.
| Marker | Fluorochrome | Volume/test (µL) |
| CD3 | mFluor™ Violet 450 | 5 |
| CD4 | mFluor™ Violet 500 | 5 |
| CD8 | Alexa Fluor® 700 | 5 |
| Live/Dead | (APC-Cy7) | 0.1 |
| LAG-3 | mFluor™ Violet 610 | 5 |
| TIM-3 | PE | 10 |
| PD-1 | Alexa Fluor® 647 | 5 |
4. (Optional) To a separate tube, add 5 μL of each of the isotype control antibodies. Vortex tubes.
5. Incubate the mixtures for 30-45 minutes at room temperature in the dark.
6. At the end of the incubation, wash with 2 mL of Staining Buffer (1X), by spinning at 300 x g for 5 minutes. Decant/aspirate supernatant.
Additional Flow Cytometry Products and Resources
Products:
MagCellect™ Cell Selection Kits
Quality Control and Standardization Beads from Novus Biologicals
Resources:
Intracellular Staining with Alcohol Permeabilization Protocol
Intracellular Staining with Detergent Permeabilization Protocol
On-Demand Webinar: Demystifying Multi-parameter Flow Cytometry
On Demand Webinar: Turning Flow Cytometry Upside Down and Inside Out