TNFR 超家族共刺激受体
TNFRSF 共刺激受体可作为免疫检查点靶标
与阻断抑制性免疫检查点受体以改善抗肿瘤免疫应答不同,研究人员也正在探寻使用共刺激免疫检查点受体的激动剂来实现这一目的。这些共刺激受体大多是肿瘤坏死因子受体超家族 (TNFRSF) 的成员。它们主要在 T 细胞、B 细胞和/或自然杀伤 (NK) 细胞表面表达,活化后,它们通过其配体或激动性抗体,可促进增强这些细胞存活、增殖和效应功能的信号通路。研究人员正在积极探索与这些受体相关的治疗的抗肿瘤作用,包括单独刺激这些受体,或者与阻断 CTLA-4 或 PD-1 等抑制受体的抗体相结合。有关目前正被作为免疫肿瘤学靶标进行研究的主要 TNFRSF 共刺激分子的详细信息,请参见以下部分。
Bio-Techne 针对作为免疫肿瘤学靶标研究的所有共刺激 TNF 受体超家族成员及其配体,提供了 R&D SystemsTM 生物活性重组蛋白。我们的产品组合包括面向各种不同的物种、带有不同标签的蛋白质,包括不断扩展的 Avi-tag 生物素化蛋白。此外,我们还提供了一系列针对这些靶标的抗体,这些抗体经过验证,可用于多种应用中,帮助我们进一步了解这些分子的免疫刺激功能。
通过 4-1BB L 活化 4-1BB 可产生 CD28 非依赖性共刺激信号
4-1BB,也称为 CD137 和 TNFRSF9,会在各种免疫细胞上广泛表达,包括活化 CD4+ 和 CD8+ T 细胞、调节性 T 细胞、自然杀伤细胞、自然杀伤 T 细胞(NKT 细胞)和树突状细胞。1-54-1BB 会以高亲和力与其配体(4-1BB 配体/TNFSF9)结合,该配体在活化巨噬细胞、B 细胞和树突状细胞上表达。6-8这一相互作用可转导一种共刺激信号,该信号可促进 CD4+ 和 CD8+ T 细胞的活化、增殖和存活,这是一种 CD28 非依赖性共刺激信号转导。8-11刺激 CD8+ T 细胞上的 4-1BB 可增强 TCR 信号转导和这些细胞的细胞毒性活性。12同样,刺激自然杀伤细胞上的 4-1BB,可增加 IFN-γ 的生成及这些细胞的细胞溶解活性。13Fc 受体发生结合后,4-1BB 在人自然杀伤细胞上上调,随后的 4-1BB 刺激通过抗体依赖性细胞介导的细胞毒性,可增强自然杀伤细胞介导的杀伤作用。14-16在树突状细胞 (DC) 上,4-1BB 会在 DC 成熟过程中强烈表达,它的刺激促进了 B7-1/CD80 和 B7-2/CD86 上调、增加了细胞因子生成并延长了生存期。3, 17
在小鼠肿瘤模型中,在使用激动性抗 4-1BB 抗体治疗后,研究人员观察到了强效的抗肿瘤免疫应答,这归结于 CD8+ T 细胞活性和自然杀伤细胞活性的增加,以及调节性 T 细胞抑制活性的降低。18-20此外,在小鼠肿瘤模型中,联合使用抗 CTLA-4 抗体和激动性抗 4-1BB 抗体的研究表明,这种联合治疗可以协同改善抗肿瘤免疫应答。21-23因此,目前人们正在研究 4-1BB 相关治疗对于几种不同的人类癌症的疗效,包括单独使用激动性抗 4-1BB 抗体,或者与激动性抗 OX40 抗体或针对负向调节免疫检查点靶标的拮抗性抗体联用。24, 25
CD70 与 CD27 结合可促进 T 细胞扩增并改善抗肿瘤免疫
CD27,也称为 TNFRSF7,主要在 T 细胞、B 细胞和自然杀伤细胞上表达。虽然在初始 CD4+ 和 CD8+ T 细胞上,CD27 仅会少量表达,但在 T 细胞活化后,其表达会上调。1, 2CD27 与 CD27 配体/CD70 结合,该配体在活化树突状细胞、B 细胞、T 细胞和自然杀伤细胞上表达,这一相互作用会产生一种与 CD28 互补的 T 细胞共刺激信号。3-9T 细胞表达的 CD27 发生结合后,可促进抗原特异性 CD4+ 和 CD8+ T 细胞的克隆扩增、支持 Th1 和 CD8+ 效应 T 细胞的分化并促进效应 T 细胞的存活。5, 10-15可溶性 CD27 也可通过从活化 T 细胞表面蛋白水解脱落 CD27 来产生。4虽然 CD27 在初始 B 细胞上不表达,但其在活化 B 细胞上的表达会上调,并且可促进效应和记忆 B 细胞的生成以及 B 细胞在生发中心的扩增。3, 4此外,CD27 会在自然杀伤细胞上表达,并且已被证明在发生结合后,可增强自然杀伤细胞增殖和 IFN‐γ 生成。2
在荷 EL-4 淋巴瘤小鼠中,研究人员证明,CD70 的转基因表达可以通过一种依赖于 CD8+ T 细胞、IFN‐γ 的方式促进肿瘤消退。16研究还表明,小鼠激动性抗 CD27 抗体在荷同源 T 细胞或 BCL1 淋巴瘤的免疫健全小鼠中具有抗肿瘤作用,在小鼠黑色素瘤模型中也得出了类似的结论。17-19同时研究也证明,在人 CD27 转基因同源小鼠肿瘤模型中,全人源激动性抗 CD27 抗体也表现出强大的抗肿瘤疗效。20当将这种人抗 CD27 抗体与抗 CTLA-4 阻断抗体联用时,可协同改善抗肿瘤免疫应答。21因此,在临床试验中,研究人员正在将激动性抗 CD27 和抗 CD70 抗体作为治疗血液系统恶性肿瘤和实体瘤的潜在方案进行研究。22, 23
通过 CD40 L 活化 CD40 可间接促进 T 细胞启动、活化和 Th1 极化
CD40,也称为 TNFRSF5,是一种 I 型跨膜受体,属于 TNF 受体超家族,在抗原呈递细胞 (APC) 表面表达,这类细胞包括树突状细胞、巨噬细胞、单核细胞和 B 细胞。1,2其配体(CD40 配体)在活化 T 细胞和 B 细胞上表达,在出现炎症的情况下,该配体也会诱导表达于自然杀伤细胞、单核细胞、嗜碱性粒细胞和肥大细胞上。3CD40 配体与 CD40 结合可促进 B 细胞活化、增殖和 T 细胞依赖性体液免疫应答。3,4此外,CD40 活化会触发相关信号通路,导致细胞因子、共刺激分子、黏附分子和酶的产生,从而影响细胞和体液免疫应答。5,6由于 CD40/CD40 配体相互作用能够影响 APC 上共刺激分子的表达及细胞因子(包括 TNF-α 和 IL-12)的生成,所以 CD40 信号转导可间接调节 T 细胞启动、T 细胞活化以及 Th1 极化。
已有研究发现,CD40 会在多种肿瘤细胞上表达,并已被证明具有环境依赖性促瘤或抑瘤作用。6虽然 CD40 和 CD40 配体在肿瘤细胞上的共表达可促进肿瘤细胞的增殖和运动,但 CD40/CD40 配体相互作用也可对肿瘤细胞产生直接的细胞毒性作用,或者通过活化 APC 间接抑制肿瘤生长。值得注意的是,在同源小鼠淋巴瘤模型中,激动性抗 CD40 抗体已被证明可刺激强效的抗肿瘤 CD8+ T 细胞应答。7-9在不同类型的晚期癌症患者中,研究人员在有关重组人 CD40 配体和激动性抗 CD40 抗体的 I 期试验中,也观察到了令人鼓舞的抗肿瘤效果。10-12当下正在开展的临床试验仍在对其中的许多激动剂进行评估,这表明刺激 CD40/CD40 配体通路对于某些癌症的治疗来说,可能是一种极富前景的未来治疗策略。13, 14
GITR L 与 GITR 结合可促进 T 细胞活化并抑制调节性 T 细胞活性
糖皮质激素诱导的 TNF 受体家族相关蛋白 (GITR),也称为 TNFRSF18,是一种共刺激 I 型跨膜受体,属于 TNF 受体超家族。它在静息 CD4+ 和 CD8+ T 细胞上以低水平表达,T 细胞活化后,表达上调。1-4GITR 也会在自然杀伤细胞上表达,并在 CD4+CD25+ 调节性 T 细胞上以高水平表达,T 细胞活化后,这一表达还会进一步上调。5-7GITR 与被称为 GITR 配体/TNFSF18 的 II 型跨膜蛋白结合。GITR 配体主要在抗原呈递细胞上表达,包括树突状细胞 (DC)、巨噬细胞和 B 细胞,并且已有研究证明,DC 细胞活化后,其在 DC 上的表达会上调。8-10GITR 配体与 GITR 结合可刺激抗 CD3 诱导的 T 细胞增殖,共同刺激 CD4+ 和 CD8+ T 细胞活化,并促进 NK 细胞活化、细胞毒性和 IFN‐γ 生成。1, 3, 4, 7, 8此外,对调节性 T 细胞上的 GITR 进行刺激,可促进细胞增殖,但会抑制细胞的抑制活性。4, 6, 11
GITR 被认为是一个极富吸引力的癌症免疫治疗靶标,因为 GITR 激动剂既可活化 CD8+ 和 CD4+ 效应 T 细胞,又能抑制调节性 T 细胞的功能。在几种同源小鼠肿瘤模型中,研究人员发现,重组小鼠 GITR 配体-Fc 融合蛋白和激动性抗 GITR 抗体均可刺激产生强效的抗肿瘤免疫应答。12-16这些激动剂的疗效归结于它们能促进 CD4+ 和 CD8+ T 细胞的肿瘤浸润增加,促进分泌 IFN‐γ 的 CD8+ T 细胞和 NK 细胞扩增,以及促进瘤内调节性 T 细胞的耗竭。12-16此外,将抗 CTLA-4 抗体和抗 GITR 抗体联用的联合治疗也显示出协同抗肿瘤活性。12, 17因此,在当下进行的 I 期临床试验中,研究人员正在对许多激动性抗 GITR 抗体进行评估。18, 19
OX40 信号转导可刺激 T 细胞活化、抑制 Treg 活性并促进肿瘤消退
OX40,也称为 TNFRSF4 和 CD134,是一种 I 型跨膜蛋白,在活化 CD4+ 和 CD8+ T 细胞、活化自然杀伤细胞、NKT 细胞以及中性粒细胞上表达上调和瞬时表达。1-4它与一种被称为 OX40 配体 (OX40 L) 的 II 型跨膜蛋白结合,该配体可在炎性细胞因子刺激下或 B 细胞受体 (BCR)、CD40 或 Toll 样受体 (TLR) 活化后,诱导表达于活化抗原呈递细胞上,这类细胞包括 B 细胞、巨噬细胞和树突状细胞 (DC)。3, 5OX40 和 OX40 L 之间的相互作用,可提供强大的 T 细胞共刺激信号,促进 T 细胞的增殖、存活及效应功能。3-5此外,在抗体与 Fc 受体结合后,OX40 信号转导可抑制调节性 T 细胞的抑制活性,刺激自然杀伤细胞的细胞毒性和 IFN-γ 的生成。2, 6, 7
研究表明,在小鼠肿瘤模型中,激动性抗 OX40 单克隆抗体和 OX40 配体-Fc 融合蛋白均可促进肿瘤消退,这归结于 T 细胞肿瘤浸润的增加,以及 CD4+ 和 CD8+ T 细胞的扩增和效应功能。8-12几项采用临床前模型的研究也表明,将抗 CTLA-4 或抗 PD-1 阻断抗体与激动性抗 OX40 抗体联用的联合治疗,可协同改善抗肿瘤免疫应答。13-15人们还在晚期癌症患者中对小鼠抗 OX40 激动性抗体进行了试验,结果显示其耐受良好,具有与先前在小鼠肿瘤模型中观察到的类似免疫刺激作用。16整体而言,种种数据表明,单独使用 OX40 激动剂,或联合抑制性免疫检查点阻断,均可能是治疗某些癌症的潜在策略。
TNF 受体超家族成员可促进 T 细胞和自然杀伤细胞活化
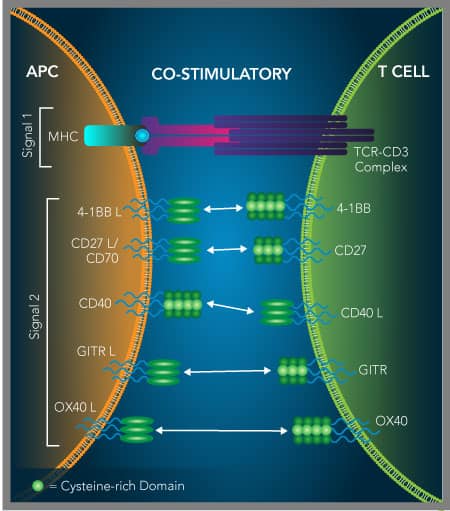
TNF 受体超家族的免疫细胞共刺激受体的活化是另一种正在被研究的癌症免疫治疗策略。作为阻断抑制性免疫检查点受体以恢复抗肿瘤免疫应答的替代方法,共刺激免疫检查点受体的激动剂也正处于探索之中。其中许多共刺激受体是 TNF 受体超家族成员,并已被证明有助于增强 T 细胞和/或自然杀伤 (NK) 细胞的增殖和效应功能,同时还有一些会抑制调节性 T 细胞的活性。
R&D Systems 重组人 4-1BB 与 4-1BB L 蛋白的结合分析
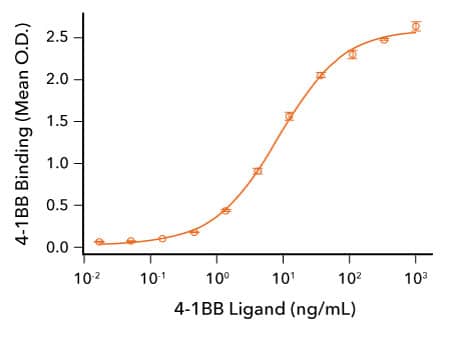
人 4-1BB 配体可与 4-1BB 结合。固定 50 ng/mL 的重组人 4-1BB/TNFRSF9(R&D Systems,目录号 9220-4B),并添加指定浓度的重组人 4-1BB 配体(R&D Systems,目录号 2295-4L)。重组人 4-1BB 配体结合的 ED50 为 2.5-15 ng/mL。
Protein Characterization Using SEC-MALS Analysis
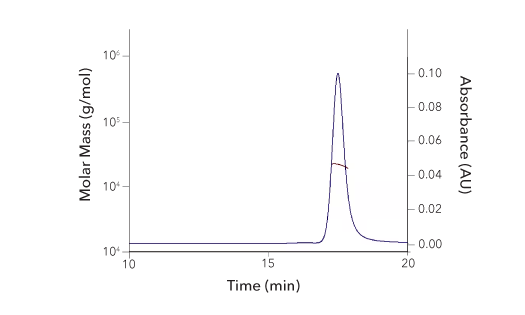
Recombinant Human 4‑1BB/TNFRSF9/CD137 Protein SEC-MALS. Recombinant Human 4-1BB/TNFRSF9 (Catalog # 9220-4B) has a molecular weight (MW) of 21.8 kDa as analyzed by SEC-MALS, suggesting that this protein is a monomer. MW may differ from predicted MW due to post-translational modifications (PTMs) present (i.e. Glycosylation).
| SEC-MALS Data | Result |
| Retention Time | 17.3-17.9 min |
| MW-Predicted (Monomer) | 18.0 kDa |
| MW-MALS | 21.8 kDa |
| Polydispersity | 1.002 |
| System Suitability: BSA Monomer 66.4 ± 3.32 kDa | Pass |
R&D Systems 重组人 CD27 配体蛋白生物活性评估

CD27 配体/CD70 可诱导人纤维肉瘤细胞中的 IL-8 分泌。用指定浓度的重组人 CD27 配体/TNFSF7(R&D Systems,目录号 9328-CL)处理经人 CD27 转染的 HT1080 人纤维肉瘤细胞。使用人 IL-8/CXCL8 QuantikineTM ELISA 试剂盒(R&D Systems,目录号 D8000C)测定细胞培养上清液中的 IL-8 分泌。这一作用的 ED50 为 5-25 ng/mL。
R&D Systems 重组人 CD40 与 CD40 L 蛋白的结合分析

CD40/TNFRSF5 可与 CD40 配体结合。固定 2 μg/mL(100 μL/孔)的重组人 CD40 配体/TNFSF5(R&D Systems,目录号 6420-CL;HEK293 细胞表达),并添加指定浓度的 Avi-tag 生物素化重组人 CD40/TNFRSF5 Fc 嵌合体(R&D Systems,目录号 AVI10380)。产生 50% 最佳结合的 Avi-tag 生物素化重组人 CD40 的浓度为 40-320 ng/mL。
R&D Systems 重组人 GITR 与 GITR L 蛋白的结合分析

GITR 可与 GITR 配体结合。固定 0.5 μg/mL(100 μL/孔)的重组人 GITR 配体/TNFSF18(R&D Systems,目录号 6987-GL),并添加指定浓度的 Avi-tag 生物素化重组人 GITR/TNFRSF18 Fc 嵌合体(R&D Systems,目录号 AVI689)。Avi-tag 生物素化重组人 GITR/TNFRSF18 结合的 ED50 为 0.05-0.5 μg/mL。
R&D Systems 重组人 OX40 蛋白结合活性和纯度评估

OX40 配体可与 OX40/TNFRSF4 结合。固定 0.25 μg/mL 的重组人 OX40/TNFRSF4(R&D Systems,目录号 9969-OX),并添加指定浓度的重组人 OX40 配体/TNFSF4(R&D Systems,目录号 1054-OX)。重组人 OX40 配体结合的 ED50 为 0.25-1.5 ng/mL。

通过 SDS-PAGE 评估重组人 OX40/TNFRSF4 的纯度。通过 SDS-PAGE 分析,在还原 (R) 和非还原 (NR) 条件下评估了重组人 OX40/TNFRSF4(R&D Systems,目录号 9969-OX)的纯度,用考马斯蓝 (Coomassie® Blue) 染色来进行可视化。
4-1BB - 4-1BB L
1. Wen, T.et al. (2002) 4-1BB ligand-mediated costimulation of human T cells induces CD4 and CD8 T cell expansion, cytokine production, and the development of cytolytic effector function. J. Immunol. 168:4897. PMID: 11994439
2. Zheng, G. et al (2004) The 4-1BB costimulation augments the proliferation of CD4+CD25+ regulatory T cells. J. Immunol. 173:2428. PMID: 15294956
3. Choi, B.K. et al (2009) 4-1BB functions as a survival factor in dendritic cells. J. Immunol. 182:4107. PMID: 19299708
4. Vinay, D.S. & B.S. Kwon:(2011) 4-1BB signaling beyond T cells. Cell. Mol. Immunol. 8:281. PMID: 21217771
5. Kim, D. et al (2008) 4-1BB engagement costimulates NKT cell activation and exacerbates NKT cell ligand-induced airway hyperresponsiveness and inflammation. J. Immunol. 180:2062. PMID: 18250411
6. Goodwin, R.G. et al (1993) Molecular cloning of a ligand for the inducible T cell gene 4-1BB: a member of an emerging family of cytokines with homology to tumor necrosis factor. Eur. J. Immunol. 23:2631. PMID: 8405064
7. Pollok, K.E. et al (1994) 4-1BB T-cell antigen binds to mature B cells and macrophages, and costimulates anti-mu-primed splenic B cells. Eur. J. Immunol. 24:367. PMID: 8299685
8. DeBenedette, M.A. et al (1997) Costimulation of CD28- T lymphocytes by 4-1BB ligand. J. Immunol. 158:551. PMID: 8992967
9. Chu, N.R. et al (1997) Role of IL-12 and 4-1BB ligand in cytokine production by CD28+ and CD28- T cells. J. Immunol. 158:3081. PMID: 9120260
10. Saoulli, K.S. et al (1998) CD28-independent, TRAF2-dependent costimulation of resting T cells by 4-1BB ligand. J. Exp. Med. 187:1849. PMID: 9607925
11. Cannons, J.L. et al (2001) 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J. Immunol. 167:1313. PMID: 11466348
12. Shuford, W.W. et al (1997) 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J. Exp. Med. 186:47. PMID: 9206996
13. Melero, I. et al (1998) NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-41BB monoclonal antibodies. Cell. Immunol. 190:167. PMID: 9878117
14. Lin, W. et al (2008) Fc-dependent expression of CD137 on human NK cells: insights into "agonistic" effects of anti-CD137 monoclonal antibodies. Blood 112:699. PMID: 18519814
15. Kohrt, H.E. et al (2011) CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood 117:2423. PMID: 21193697
16. Wang, W. et al (2015) NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front. Immunol. 6:368. PMID: 26284063
17. Kuang, Y. et al (2012) Effects of 4-1BB signaling on the biological function of murine dendritic cells. Oncol. Lett. 3:477. PMID: 22740935
18. Melero, I. et al (1997) Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat. Med. 3:682. PMID: 9176498
19. Xu, D. et al (2004) NK and CD8+ T cell-mediated eradication of poorly immunogenic B16-F10 melanoma by the combined action of IL-12 gene therapy and 4-1BB costimulation. Int. J. Cancer 109:499. PMID: 14991570
20. Buchan, S.L.et al. (2018) Antibodies to costimulatory receptor 4-1BB enhance anti-tumor immunity via T regulatory cell depletion and promotion of CD8 T cell effector function. Immunity 49:958. PMID: 30446386
21. Kocak, E. et al (2006) Combination therapy with anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity. Cancer Res. 66:7276. PMID: 16849577
22. Li, B. et al (2007) Established B16 tumors are rejected following treatment with GM-CSF-secreting tumor cell immunotherapy in combination with anti-4-1BB mAb. Clin. Immunol. 125:76. PMID: 17706463
23. Curran, M. et al (2011) Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS One 6:19499. PMID: 21559358
24. Dempke, W.C.M. et al (2017) Second- and third-generation drugs for immuno-oncology treatment-the more the better? Eur. J. Cancer 74:55. PMID: 28335888
25. Marin-Acevedo, J.A.:(2018) Next generation of immune checkpoint therapy in cancer: new developments and challenges. J. Hematol. Oncol. 11:39. PMID: 29544515
CD27- CD27 L/CD70
1. Loenen, W.A.et al. (1992) The CD27 membrane receptor, a lymphocyte-specific member of the nerve growth factor receptor family, gives rise to a soluble form by protein processing that does not involve receptor endocytosis. Eur. J. Immunol. 22:447. PMID: 1311261
2. Takeda, K. et al (2000) CD27-mediated activation of murine NK cells. J. Immunol. 164:1741. PMID: 10657619
3. Lens, S.M. et al (1998) Control of lymphocyte function through CD27-CD70 interactions. Semin. Immunol. 19:491. PMID: 9826582
4. Borst, J. et al (2005) CD27 and CD70 in T cell and B cell activation. Curr. Opin. Immunol. 17:275. PMID: 15886117
5. Goodwin, R.G. et al (1993) Molecular and biological characterization of a ligand for CD27 defines a new family of cytokines with homology to tumor necrosis factor. Cell 73:447. PMID: 8387892
6. Bowman, M.R. et al (1994) The cloning of CD70 and its identification as the ligand for CD27. J. Immunol. 152:1756. PMID: 8120384
7. Hintzen, R.Q. et al (1994) CD70 represents the human ligand for CD27. Int. Immunol. 6:477. PMID: 8186199
8. Tesselaar, K. et al (2003) Expression of the murine CD27 ligand CD70 in vitro and in vivo. J. Immunol. 170:33. PMID: 12496380
9. Nolte, M.A. et al (2009) Timing and tuning of CD27-CD70 interactions: the impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunol. Rev. 229:216. PMID: 19426224
10. Van Oosterwijk, M.F. et al (2007) CD27-CD70 interactions sensitise naive CD4+ T cells for IL-12-induced Th1 cell development. Int. Immunol. 19:713. PMID: 17548342
11. Xiao, Y. et al (2008) CD27 instructs CD4+ T cells to provide help for the memory CD8+ T cell response after protein immunization. J. Immunol. 181:1071. PMID: 18606659
12. Rowley, T.F. & A.A. Shamkhani:(2004) Stimulation by soluble CD70 promotes strong primary and secondary CD8+ cytotoxic T cell responses in vivo. J. Immunol. 172:6039. PMID: 15128787
13. Van Gisbergen, K. et al (2011) The costimulatory molecule CD27 maintains clonally diverse CD8(+) T cell responses of low antigen affinity to protect against viral variants. Immunity 35:97. PMID: 21763160
14. Peperzak, V. et al (2010) The Pim kinase pathway contributes to survival signaling in primed CD8+ T cells upon CD27 costimulation. J. Immunol. 185:6670. PMID: 21048108
15. Pen, J.J. et al (2013) Modulation of regulatory T cell function by monocyte-derived dendritic cells matured through electroporation with mRNA encoding CD40 ligand, constitutively active TLR4, and CD70. J. Immunol. 191:1976. PMID: 23842750
16. Arens, R. et al (2004) Tumor rejection induced by CD70-mediated quantitative and qualitative effects on effector CD8+ T cell formation. J. Exp. Med. 199:1595. PMID: 15184507
17. Sakanishi, T. & H. Yagita:(2010) Anti-tumor effects of depleting and non-depleting anti-CD27 monoclonal antibodies in immune-competent mice. Biochem. Biophys. Res. Commun. 393:829. PMID: 20171165
18. French, R.R. et al (2007) Eradication of lymphoma by CD8 T cells following anti-CD40 monoclonal antibody therapy is critically dependent on CD27 costimulation. Blood 109:4810. PMID: 17311995
19. Roberts, D.J. et al (2010) Control of established melanoma by CD27 stimulation is associated with enhanced effector function and persistence, and reduced PD-1 expression of tumor infiltrating CD8(+) T cells. J. Immunother. 33:769. PMID: 20842060
20. He, L-Z. et al (2013) Agonist anti-human CD27 monoclonal antibody induces T cell activation and tumor immunity in human CD27-transgenic mice. J. Immunol. 191:4174. PMID: 24026078
21. He, L-Z. et al (2013) Combination therapies augment the anti-tumor activity of agonist CD27 mAb in human CD27 transgenic mouse models. J. Immunother. Cancer 1:76. PMID:
22. Dempke, W.C.M. et al (2017) Second- and third-generation drugs for immuno-oncology treatment-the more the better? Eur. J. Cancer 74:55. PMID: 28335888
23. Marin-Acevedo, J.A.:(2018) Next generation of immune checkpoint therapy in cancer: new developments and challenges. J. Hematol. Oncol. 11:39. PMID: 29544515
CD40 - CD40 L
1. van Kooten, C. & J. Banchereau:(1997) Functions of CD40 on B cells, dendritic cells and other cells. Curr. Opin. Immunol. 9:330. PMID: 9203418
2. Schonbeck, U. et al (1997) Ligation of CD40 activates interleukin 1beta-converting enzyme (caspase-1) activity in vascular smooth muscle and endothelial cells and promotes elaboration of active interleukin 1beta. J. Biol. Chem. 272:19569. PMID: 9235962
3. Elgueta, R. et al (2009) Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 229:152. PMID: 19426221
4. Rickert, R.C. et al (2011) Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol. Rev. 244:115. PMID: 22017435
5. Schonbeck, U. et al (2001) The CD40/CD154 receptor/ligand dyad. Cell. Mol. Life Sci. 58:4. PMID: 11229815
6. Kawabe, T. et al (2011) CD40/CD40 ligand interactions in immune responses and pulmonary immunity. Nagoya J. Med. Sci. 73:69. PMID: 21928689
7. van Kooten, C. & J. Banchereau:(2000) CD40-CD40 ligand. J. Leukoc. Biol. 67:2. PMID: 10647992
8. French, R.R. et al (1999) CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat. Med. 5:548. PMID: 10229232
9. Tutt, A.L. et al (2002) T cell immunity to lymphoma following treatment with anti-CD40 monoclonal antibody. J. Immunol. 168:2720. PMID: 11884438
10. Vonderheide, R.H. et al (2001) Phase I study of recombinant human CD40 ligand in cancer patients. J. Clin. Oncol. 19:3280. PMID: 11432896
11. Vonderheide, R.H. et al (2007) Prospect of targeting the CD40 pathway for cancer therapy. Clin. Cancer Res. 13:1083. PMID: 17317815
12. Vonderheide, R.H. et al (2013) Agonistic CD40 antibodies and cancer therapy. Clin. Cancer Res. 19:1035. PMID: 23460534
13. Dempke, W.C.M. et al (2017) Second- and third-generation drugs for immuno-oncology treatment-the more the better? Eur. J. Cancer 74:55. PMID: 28335888
14. Marin-Acevedo, J.A.:(2018) Next generation of immune checkpoint therapy in cancer: new developments and challenges. J. Hematol. Oncol. 11:39. PMID: 29544515
GITR - GITR L
1. Nocentini, G.et al. (1997) A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc. Natl. Acad. Sci. USA 94:6216. PMID: 9177197
2. Kwon, B. et al (1999) Identification of a novel activation-inducible protein of the tumor necrosis factor receptor superfamily and its ligand. J. Biol. Chem. 274:6056. PMID: 10037686
3. Gurney, A.L. et al (1999) Identification of a new member of the tumor necrosis factor family and its receptor, a human ortholog of mouse GITR. Curr. Biol. 9:215. PMID: 10074428
4. Ronchetti, S. et al (2004) GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur. J. Immunol. 34:613. PMID: 14991590
5. Shimizu, J. et al (2002) Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 3:135. PMID: 11812990
6. Kanamaru, F. et al (2004) Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. J. Immunol. 172:7306. PMID: 15187106
7. Hanabuchi, S. et al (2006) Human plasmacytoid predendritic cells activate NK cells through glucocorticoid-induced tumor necrosis factor receptor-ligand (GITRL). Blood 107:3617. PMID: 16397134
8. Tone, M. et al (2003) Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc. Natl. Acad. Sci, USA 100:15059. PMID: 14608036
9. Kim, J.D. et al (2003) Cloning and characterization of GITR ligand. Genes Immunol. 4:564. PMID: 14647196
10. Yu, K. et al (2003) Identification of a ligand for glucocorticoid-induced tumor necrosis factor receptor constitutively expressed in dendritic cells. Biochem. Biophys. Res. Commun. 310:433. PMID: 14521928
11. McHugh, R.S. et al (2002) CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity 16:311. PMID: 11869690
12. Hu,, P. et al (2008) Construction and preclinical characterization of Fc-mGITRL for the immunotherapy of cancer. Clin. Cancer Res. 14:579. PMID: 18223234
13. Ko, K. et al (2005) Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating FoxP3+CD25+CD4+ regulatory T cells. J. Exp. Med. 202:885. PMID: 16186187
14. Zhou, P. et al (2007) Pivotal roles of CD4+ effector T cells in mediating agonistic anti-GITR mAb-induced immune activation and tumor immunity in CT26 tumors. J. Immunol. 179:7365. PMID: 18025180
15. Coe, D. et al (2010) Depletion of regulatory T cells by anti-GITR mAb as a novel mechanism for cancer immunotherapy. Cancer Immunol. Immunother. 59:1367. PMID: 20480365
16. Cohen, A.D. et al (2010) Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS One 5:10436. PMID: 20454651
17. Mitsui, J. et al (2010) Two distinct mechanisms of augmented antitumor activity by modulation of immunostimulatory/inhibitory signals. Clin. Cancer Res. 16:2781. PMID: 20460483
18. Dempke, W.C.M. et al (2017) Second- and third-generation drugs for immuno-oncology treatment-the more the better? Eur. J. Cancer 74:55. PMID: 28335888
19. Marin-Acevedo, J.A. et al (2018) Next generation of immune checkpoint therapy in cancer: new developments and challenges. J. Hematol. Oncol. 11:39. PMID: 29544515
OX40 - OX40 L
1. Gramaglia, I. et al. (1998) OX-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J. Immunol. 161:6510. PMID: 9862675
2. Turaj, A.H. et al (2018) Augmentation of CD134 (OX40)-dependent NK anti-tumour activity is dependent on antibody cross-linking. Sci. Rep. 8:2278. PMID: 29396470
3. Croft, M.:(2010) Control of immunity by the TNFR-related molecule OX40 (CD134). Ann. Rev. Immunol. 28:57. PMID: 20307208
4. Willoughby, J. et al (2017) OX40: Structure and function - What questions remain? Mol. Immunol. 83:13. PMID: 28092803
5. Mendel, I. & E.M. Shevach:(2005) Activated T cells express the OX40 ligand: requirements for induction and costimulatory function. Immunology 117:196. PMID: 16423055
6. Takeda, I. et al (2004) Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J. Immunol. 172:3580. PMID: 15004159
7. Aspeslagh, S. et al (2016) Rationale for anti-OX40 cancer immunotherapy. Eur. J. Immunol. 52:50. PMID: 26645943
8. Weinberg, A.D. et al (2000) Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J. Immunol. 164:2160. PMID: 10657670
9. Kjaergaard, J. et al (2000) Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 60:5514. PMID: 11034096
10. Ali, S.A. et al (2004) Anti-tumor therapeutic efficacy of OX40L in murine tumour model. Vaccine 22:3585. PMID: 15315837
11. Gough, M.J. et al (2008) OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Res. 68:5206. PMID: 18593921
12. Piconese, S. et al (2008) OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J. Exp. Med. 205:825. PMID: 18362171
13. Redmond, W.L. et al (2014) Combined targeting of costimulatory (OX40) and coinhibitory (CTLA-4) pathways elicits potent effector T cells capable of driving robust antitumor immunity. Cancer Immunol. Res. 2:142. PMID: 24778278
14. Marabelle, A. et al (2013) Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J. Clin. Invest. 123:2447. PMID: 23728179
15. Guo, Z. et al (2014) PD-1 blockade and OX40 triggering synergistically protects against tumor growth in a murine model of ovarian cancer. PLoS One 9:89350. PMID: 24586709
16. Curti, B.D. et al (2013) OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 73:7189. PMID: 24177180