By Victoria Osinski, PhD
Despite advances in the development of cancer immunotherapies, treatments specifically targeting tumors remain limited. Utilizing chimeric antigen receptor (CAR) T cell therapies, a strategy that utilizes patients’ T cells and genetically engineers them to produce a T cell receptor to mount an attack against cancer cells, has generated great interest. While CAR T cells can effectively kill cancer cells in tumors, they often come with off-tumor effects because the target antigens of CARs are found endogenously in the body. Tumors are typically hypoxic, meaning they lack sufficient oxygen levels, due to rapid tumor cell expansion, dysregulated vasculature, and altered metabolism.1 This characteristic is unique to tumors and thus can be harnessed as a promising tumor-specific target for CAR T cell development. A team of researchers at King’s College London has created a new CAR T cell capable of specifically targeting hypoxic solid tumors in mice.2,3 This development should help address one of the challenges in developing CAR T cells: reducing off-tumor effects that can lead to toxicity.
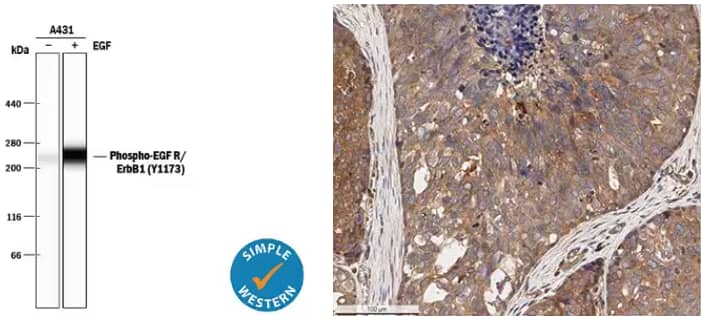
Left: Simple Western lane view analysis of lysates from A431 human epithelial carcinoma cell line either untreated (-) or treated (+) with 10 ng/mL Recombinant Human EGF (Catalog # 236-EG). Lysates were probed with Rabbit Anti-Human Phospho-EGFR (Y1173) Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1095) and Phospho-EGFR (Y1173) was detected at ~265 kDa (as indicated by the specific band). The experiment was performed under reducing conditions on the 66-440 kDa separation system. Right: Immunohistochemical (IHC) detection of ErbB2 in formalin-fixed paraffin embedded sections of human breast carcinoma tissue using Rabbit Anti-Human ErbB2/Her2 Polyclonal Antibody (Catalog # NBP2-29624) on a Bond Rx autostainer (Leica Biosystems). Signal was detected with Bond Polymer Refine Detection (Leica Biosystems) and DAB followed counterstaining with Hematoxylin. ErbB2 antibody staining revealed diffuse cytoplasmic and some membrane and nuclear staining in the cancer cells, endothelial cells, and tumor stromal cells. The cells at the core of some tumor areas were negative for ErbB2.
Using Tumor Properties to Design CAR T cells
The initial hypoxia-specific CAR T cell was developed using the second-generation pan-anti-ErbB CART1E28z T4-CAR. This construct results in two chimeric receptors: the T1E28z, which targets 8 of 9 ErbB receptors in both humans and mice, and the T4-CAR, which promotes ex vivo T cell expansion without affecting CAR T cell killing capacity in vivo. ErbB proteins are a family of receptor tyrosine kinases (RTKs) that include EGFR (ErbB1), HER2 (ErbB2), HER3 (ErbB3), and HER4 (ErbB4). Through mutations or overexpression, this family of receptors is often found and involved in a variety of cancers, including solid tumors.4 Targeting ErbB receptors is challenging, however, because these receptors are expressed in healthy tissues as well as tumors. Indeed, this study demonstrated that IV infusion of pan-anti-ErbB CART1E28z, T4-CAR is toxic in mice leading to weight loss and off-tumor effects in the liver and lungs. While intratumoral delivery caused fewer side effects, this localized approach does not allow for targeting of metastatic tumors.
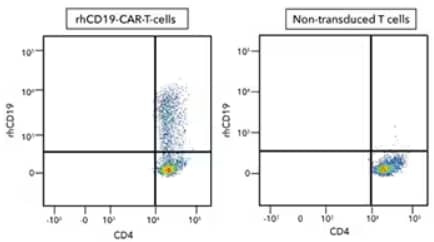
Human peripheral blood mononuclear cell (PBMC) CD4+ and CD8+ T cells that were either (left) transduced or (right) not transduced with human CD19-CAR and then cultured for 11 days. Cells were then stained with a Human CD4 PE-Cy7-conjugated Antibody and Recombinant Human CD19 Fc Chimera Atto 647N Protein (Catalog # ATM9269) and detected by flow cytometry analysis.
HypoxiCAR: Harnessing Hypoxia in CAR T cells
To utilize systemic administration but limit off-tumor effects, the researchers chose to exploit the high levels of hypoxia within solid tumors. The research group added two components to the next generation anti-ErbB CART1E28z, T4-CAR to ensure it is expressed only under hypoxic conditions. The first addition was an oxygen-dependent degradation domain (ODD) to target the transcription factor for degradation in normoxic (normal oxygen level) conditions. The ODD is one mechanism by which hypoxia-inducible factor-1 alpha (HIF-1α) is targeted for degradation. This ODD-modified CAR was originally generated by another team of researchers.5 Unfortunately, the investigators still found tumor-killing in normoxic environments when the ODD was added to the CAR. To ensure a more tightly, hypoxia-regulated system, the researchers added hypoxia-responsive elements (HREs) to an enhancer region of the CAR’s promoter. While the HREs promote gene expression only in hypoxic environments, expression of the T4-CAR was still found in normoxic environments due to the lack of the ODD in this receptor allowing for efficient expansion in normal oxygen levels ex vivo. Most importantly, this new design, known as HypoxiCAR, specifically upregulated CAR expression in hypoxic environments and prevented tumor growth in vivo, while avoiding untoward effects.
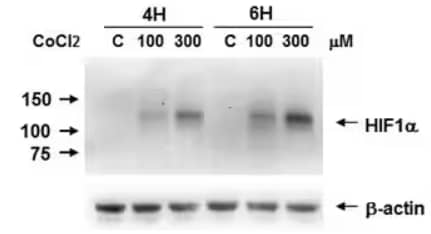
Biological Strategies Validation. Western Blot Analysis of Caki-1 cell lysates either untreated (C) or treated with CoCl2 (100 μM, 300 μM) for 4 or 6 hours to induce HIF-1α expression which was detected using Mouse Anti-HIF-1 alpha Monoclonal Antibody (H1alpha67) (Catalog # NB100-105). Image submitted by a verified customer review.
In summary, this study presents a novel hypoxia-responsive CAR T cell that is a promising opportunity to further advance the field of immunotherapies for solid tumors. Designing non-surgical approaches for treating solid tumors and potentially preventing metastasis will be valuable if they can be proven safe and effective in patients.
Note:
Kotsi, P. et al (2021) used Goat Anti-EGF Polyclonal Antibody [Biotin] (Catalog # BAF236), Mouse Anti-ErbB4/Her4 Monoclonal Antibody (H4.77.16 (Ab77)) (Catalog # NB120-3104), and Human IFN-Gamma DuoSet ELISA Kit (Catalog # DY285B).

Postdoctoral Research Fellow, University of Minnesota Medical School
-
Muz, B. et al. (2015) The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy Hypoxia (Auckl) 3:83-92.
-
Kosti, P. et al. (2021) Hypoxia-sensing CAR T cells provide safety and efficacy in treating solid tumors Cell Rep Med
-
Kosti, P. et al. (2021) Generation of hypoxia-sensing chimeric antigen receptor T cells STAR Protoc
-
Appert-Collin, A. et al. (2015) Role of ErbB Receptors in Cancer Cell Migration and Invasion Front Pharmacol
-
Juillerat, A. et al. (2017) n oxygen sensitive self-decision making engineered CAR T-cell Sci Rep