Accelerating Gene Therapy: Precise Potency and Transgene Expression with Simple Western
Simple Western technology sets the standard for quantitative, reproducible, and precise analytical-grade protein expression potency assays with size-based specificity. It provides a multi-attribute platform for cell and gene therapy process development, enabling quantitative monitoring of critical quality attributes (CQAs) such as purity, titer, empty/full capsid content, and capsid protein ratios. Simple Western is a fully automated capillary electrophoresis (CE) platform that pairs protein size separation with a quantitative immunoassay readout—delivering high-quality data with minimal hands-on time.
- Quantitation – Achieve precise protein measurements with a 4-log dynamic range.
- Sensitivity – Conserve precious adeno-associated virus (AAV) samples while ensuring robust CQA analysis.
- Reproducibility – Eliminate variability inherent in traditional Western blots, with CVs below 15-20%.
- High Throughput – Analyze up to 96 data points in a fully automated 3-hour run.
- Method Transferability – Seamlessly transfer methods between sites or from sponsors to CROs, with 21 CFR Part 11 compliance and GMP-ready workflows.
Fast, Precise, and Reproducible AAV Potency Testing for Gene Therapy Development
- Leo delivers precise, analytical-grade quantification of relative potency for gene therapy assays by processing up to 96 unique samples in a single run—with ample space for replicates and controls.
- Whether measuring potency using a 7-point titration curve or quantifying protein expression with a 12-point standard curve, Leo ensures highly sensitive, reproducible data in a streamlined 3-hour workflow.
- This high-throughput capability allows researchers to assess batch-to-batch consistency, stress-induced potency changes, and critical quality attributes (CQAs) like capsid ratios, purity, and empty/full content with confidence.
- By reducing hands-on time and increasing data accuracy, Leo accelerates gene therapy development with the precision required for regulatory success.
- Your existing Simple Western assay can be easily transferred to Leo with consistent results
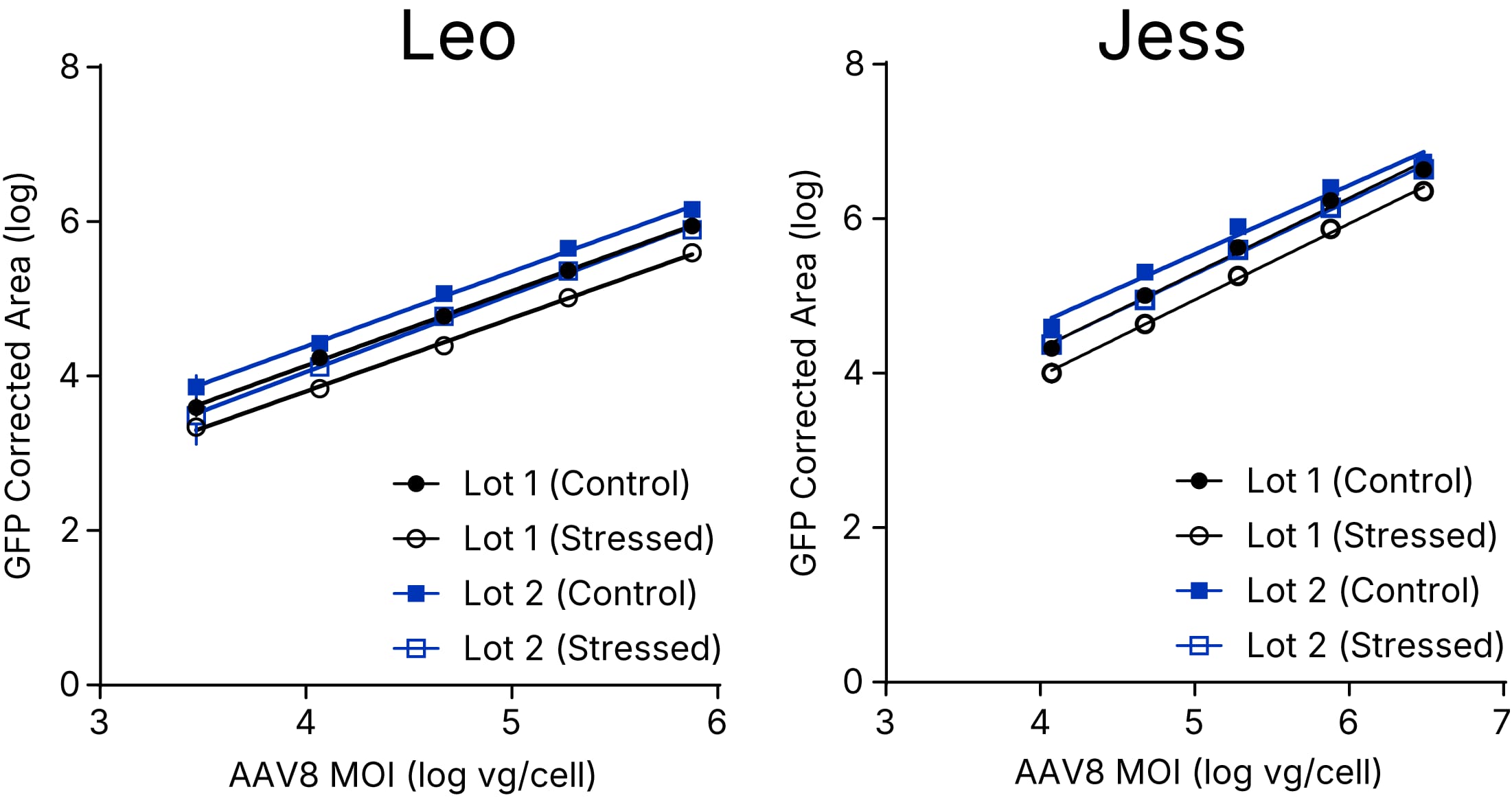
Relative potency measurements of 2 lots of AAV8 (stressed and non-stressed) using Leo & Jess
The Simple Way to Advance Gene Therapy
In this webinar, we show how Leo has the potential to transform western blots into quantitative bioanalytical methods suitable for adoption for robust quantitative measures of protein expression, including gene therapy potency measurements.
Simple Western Assays for Gene Therapy
Potency Assay
The need for fit-for-purpose potency assays is a major challenge preventing transformative gene therapies from reaching patients. These assays are some of the most important in an analytical package but are often the most complex and high-risk. Potency assays must be quantitative, dose-responsive, and well-controlled. Because of their complex mechanism of action, potency assays require a matrix approach of multiple complementary assays, incorporating infectivity, transgene mRNA and protein expression, and biological activity.
Simple Western technology provides fit-for-purpose protein expression potency assays, enabling efficient, simple, and inexpensive upstream and downstream process development. It quantifies analytical-grade protein expression for relative potency assays in a multi-attribute platform approach. Detection of AAVs in transduced cells serves as a surrogate for infectivity.
Key assay data:
(1) Relative protein expression potency measurements between AAV serotypes using parallel line analysis (PLA)
(2) Absolute protein expression measurements using a recombinant standard curve
(3) Quantification of AAV VP1/VP2/VP3 capsid proteins in transduced cells as a surrogate for AAV infectivity

Simple Western relative potency assay for AAV-mediated gene delivery in cultured human cells.
Viral Identity and Purity Assay
Identity and purity are CQAs monitored during AAV manufacturing. While ELISA can provide identity information, it cannot provide purity information in the same assay. By contrast, Simple Western is a multi-attribute method delivering both identity and purity in the same assay. For example, antibodies specific to VP1/VP2/VP3 capsid proteins provide identity measurements, and total protein detection provides purity measurements. The 5X biotin labeling reagent provides ultrasensitive and reproducible total protein measurements in the upper picogram range, rivaling the best gel staining techniques like SYPRO Ruby.
Key assay data:
(1) 5X biotin labeling reagent increases DnaK detection sensitivity
(2) RNase A spiked into purified AAV sample and analyzed with 1X and 5X total protein labeling reagent on Jess
(3) Multi-attribute identity and purity measurements of AAVs using RePlex
(4) Comparison of 5X biotin labeling reagent to SYPRO Ruby

Identity is provided by an anti-VP1/2/3 antibody (green), and purity by total protein (grey).
Empty/Full Content Ratio
Simple Western provides automated empty/full AAV capsid content ratio measurements with high sensitivity, reproducibility, and scalability. Simple Western is a multi-attribute platform method for characterizing multiple AAV serotypes in complex sample types.
Key assay data:
(1) Empty/full content ratio of AAVs and assay linearity, reproducibility, and specificity
(2) Platform method approach for multiple AAV serotypes
(3) Empty/full measurements of intact AAVs using Simple Western Charge as an orthogonal method

The simultaneous detection of VP1/2/3 and DNA content for quantification of the % full AAVs.
Viral Titer and VP Capsid Protein Ratio Measurements
Comprehensive monitoring of VP1, VP2, and VP3 protein concentrations and their molar ratios is an effective tool to ensure initial optimization and consistent quality of the final AAV gene therapy products. Here, we introduce PROGEN's recombinant VP standards, used with Simple Western automated CE analysis in fit-for-purpose solutions for viral titer and VP capsid protein ratio measurements. PROGEN’s recombinant AAV2 VP protein standards are helpful for accurately and sensitively analyzing AAVs' VP content and VP ratios.
Key assay data:
(1) Establishment of an AAV2 VP1, VP2, and VP3 Protein Standard (PROGEN)
(2) Analysis of VP1, VP2, and VP3 for capsid protein ratio and titer measurements
(3) Demonstration of Simple Western reproducibility and linearity and comparison to traditional Western blot

VP molar ratios are accurately quantified for optimization of AAV gene therapies.
Process-Related Impurities
Highly sensitive Simple Western immunoassays accurately detect residual proteins resulting from therapeutic protein and vaccine development processes like host cell protein (HCP) contamination, Protein A, GFP, and bovine serum albumin (BSA).
This assay focuses on the accurate detection of four candidate contaminants that may be present during various stages of the therapeutic protein and vaccine development processes: host cell protein (HCP), Protein A, green fluorescence protein (GFP), and bovine serum albumin (BSA).

HEK293 HCP measurements by an anti-HEK HCP antibody (top) and by total protein stain (bottom).
Simple Western Assays for Immune Cell Therapy
Vector Analysis
The Simple Western automated multi-attribute platform provides scalable at-line lentiviral analytics, including p24 titer, identity, purity, stability, capsid content ratio, and transduction measurements.
Key assay data:
(1) p24 titer measurements and cross-reactivity with other lentiviral proteins
(2) Lentiviral empty/full capsid content and stability assays
(3) 5X total protein labeling reagent versus anti-HEK293T HCP antibody
(4) Lentiviral transduction of iPSC-derived macrophages (customer data)

Automated titer assays with linearity and high sensitivity optimized for AAV and lentivirus.
CAR-T Cell Signaling and Activation
Central to engineering effective CAR-T cell therapies is a detailed understanding of the signaling cascades that regulate CAR-T cells, including trafficking and tumor infiltration, preventing antigen escape, resisting immunosuppressive responses, and ameliorating potentially fatal toxicity. Detailed characterization of extracellular, membrane-bound, and intracellular signaling molecules is required, often with limited and complex sample types.
In this App Note, we demonstrate a streamlined workflow for engineering, identifying, and characterizing CAR-T cell signaling and activation status. This reveals the kinetics of CAR-T cell activation and signal transduction with reproducible quantification. The Simple Western platform monitored intracellular signaling events with high-quality antibodies from CST.
Analytical Tools to Evaluate CAR-T Cell Signaling & Activation

Quantification of phospho/total isoform ratios of CAR-T cell signaling proteins.
Watch How Simple Western Advances Cell and Gene Therapy
Overcoming Challenges in Gene Therapy Analytics
Watch now and learn about three critical aspects of viral vector characterization:
- Charge Heterogeneity of Individual Capsid Proteins: Learn how the charge heterogeneity of individual capsid proteins can be critical for developing effective viral vector-based therapies.
- Standardizing Methods to Determine Full-to-Empty Ratios: Discover the latest techniques and best practices for accurately determining full-to-empty ratios, ensuring consistent and efficient gene therapy manufacturing.
- Characterizing Vector Purity Using CE: Explore an automated CE-SDS method and gain insight into studying AAV sample impurities, including protein detection and encapsulated/free DNA, for gene therapy production.
Dystrophin Quantification in Preclinical and Clinical Settings
Duchenne muscular dystrophy (DMD) results from mutations in the dystrophin encoding DMD gene that disrupt the reading frame. Muscle fibers lacking dystrophin are more sensitive to damage. Over time, muscle tissue and muscle function diminish progressively, resulting in wheelchair dependency around age ten and premature death in the 2nd to 4th decade of life. Mutations that maintain the reading frame allow the production of internally deleted partially functional dystrophins. These mutations are associated with the later onset, less progressive Becker muscular dystrophy. This reading frame rule forms the premise of current dystrophin-restoring therapies, such as exon skipping to allow Duchenne patients to produce Becker-type dystrophin and gene therapy to deliver a micro-dystrophin transgene to muscles.
In this webinar with Dr. Annemieke Aartsma-Rus, Professor of Translational Genetics at Leiden University Medical Center, you will learn the different approaches and considerations to the question of how much dystrophin is enough, how to measure this in clinical trial settings, and how Simple Western's capillary immunoassay can be used for analyzing dystrophin in preclinical studies.
Establishment of AAV2 VP Protein Standards and their Use in CE
AAVs represent the most commonly used viral vector for delivering therapeutic transgenes. However, the gene therapy community faces significant challenges, such as the high demand for efficient production of high-quality AAV vectors in clinical-scale quantities.
To address these challenges, robust, quality protein standards and fast, automated, highly sensitive analytical instrumentation are needed. Comprehensive monitoring of VP1, VP2, and VP3 protein concentrations and their molar ratios is an effective tool to ensure initial optimization and consistent quality of the final AAV gene therapy products.
Here, we introduce PROGEN's recombinant VP standards, used with Simple Western automated CE analysis in fit-for-purpose solutions for viral titer and VP capsid protein ratio measurements.
Get Your Therapies to Market Faster
Quick and Accurate Testing for Lentivirus and AAVs
This technical seminar will teach you how Simple Western technology can improve your vector analytics. Simple Western instruments are multi-attribute machines for both lentivirus and AAVs. They provide rapid assessment of viral titer and impurity analysis for both lentivirus and AAV. Plus, they provide insight into capsid protein ratio and empty/full content ratio for AAVs. You will discover how Simple Western can easily fit into your current workflows and adapt to your changing needs, including analysis of newer viral vector modalities like retroviruses. You will also learn how our Simple Plex and Maurice platforms and Simple Western can provide a complete solution for your viral vector analytics.
