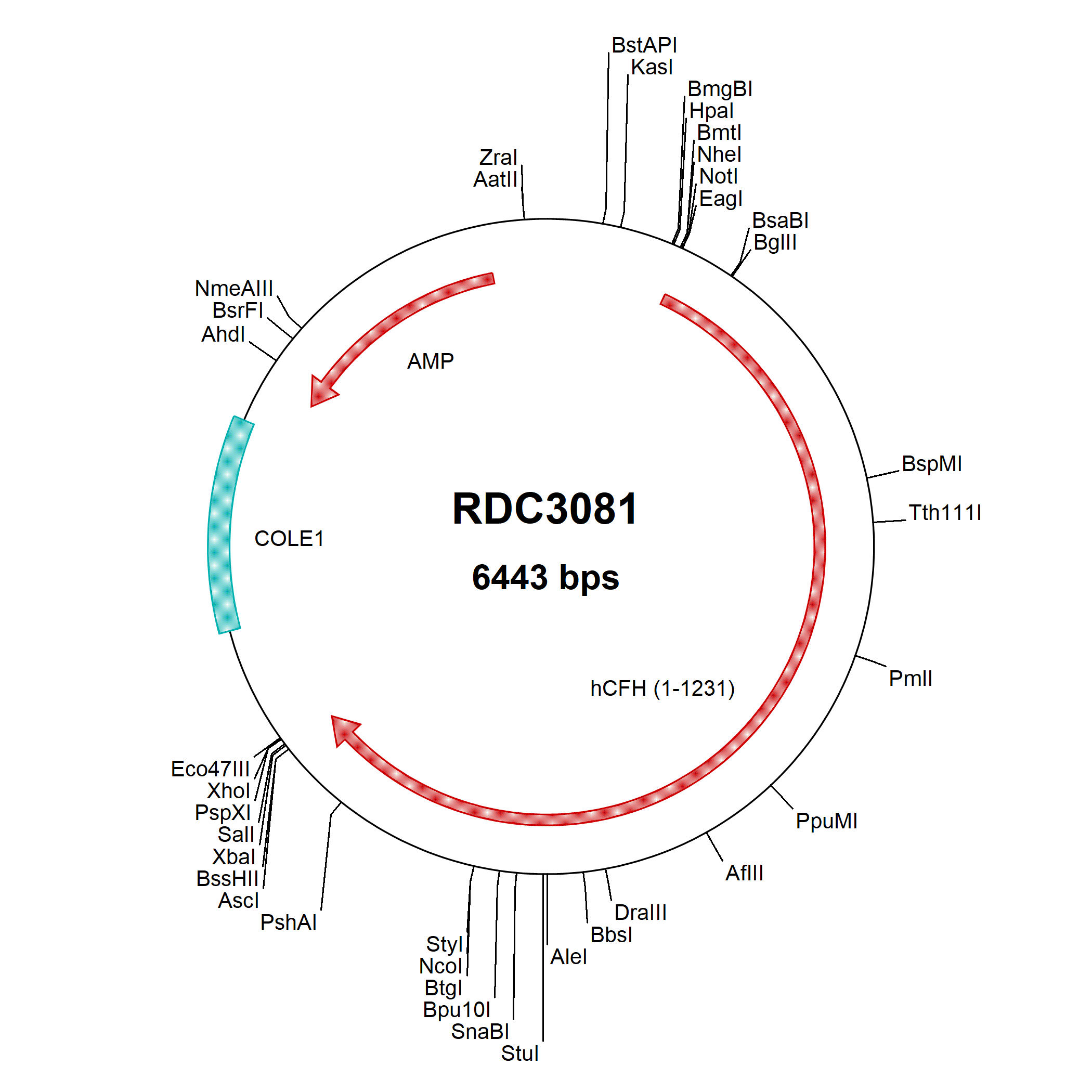
Complement Factor H: cDNA Clones
Complement Factor H is a 155 kDa glycoprotein that provides critical negative regulation to the alternative pathway of complement cascade. It is secreted by Kupffer cells, hepatocytes, vascular endothelial cells, and platelets, and circulates in the serum at high concentration. Complement Factor H is composed of 20 SCRs (short consensus repeats), each of which consists of approximately 60 amino acids with four invariant Cys residues. Alternate splicing generates a Complement Factor H isoform that is truncated following SCR7.
Complement Factor H interacts with cell surface polyanions including heparin and sialoglycoproteins, and immobilized Complement Factor H supports the CD11b/CD18 integrin-dependent adhesion of neutrophils. It prevents local complement activation by sequestering complement component C3b, accelerating the decay of C3 and C5 convertases, and functions as a cofactor for the C3b inactivator, Factor I.
Short consensus repeats 15-20 (SCR15-20) encompass the primary binding sites for heparin and C3b, as well as for the peptide hormone adrenomedullin. Within SCR15-20, human Complement Factor H shares 60% and 63% amino acid sequence identity with mouse and rat Complement Factor H, respectively. Dozens of mutations clustered in SCR15-20 are associated with atypical hemolytic uremic syndrome, a disorder characterized by anemia, thrombocytopenia, and renal failure. Binding of Complement Factor H to tumor cell-associated dentin matrix protein 1, bone sialoprotein, or osteopontin results in the protection of that cell from complement-mediated lysis. A variety of pathogenic microbes also express Complement Factor H binding molecules that interfere with immune clearance of the infection.
1 result for "Complement Factor H cDNA Clones" in Products
1 result for "Complement Factor H cDNA Clones" in Products
Complement Factor H: cDNA Clones
Complement Factor H is a 155 kDa glycoprotein that provides critical negative regulation to the alternative pathway of complement cascade. It is secreted by Kupffer cells, hepatocytes, vascular endothelial cells, and platelets, and circulates in the serum at high concentration. Complement Factor H is composed of 20 SCRs (short consensus repeats), each of which consists of approximately 60 amino acids with four invariant Cys residues. Alternate splicing generates a Complement Factor H isoform that is truncated following SCR7.
Complement Factor H interacts with cell surface polyanions including heparin and sialoglycoproteins, and immobilized Complement Factor H supports the CD11b/CD18 integrin-dependent adhesion of neutrophils. It prevents local complement activation by sequestering complement component C3b, accelerating the decay of C3 and C5 convertases, and functions as a cofactor for the C3b inactivator, Factor I.
Short consensus repeats 15-20 (SCR15-20) encompass the primary binding sites for heparin and C3b, as well as for the peptide hormone adrenomedullin. Within SCR15-20, human Complement Factor H shares 60% and 63% amino acid sequence identity with mouse and rat Complement Factor H, respectively. Dozens of mutations clustered in SCR15-20 are associated with atypical hemolytic uremic syndrome, a disorder characterized by anemia, thrombocytopenia, and renal failure. Binding of Complement Factor H to tumor cell-associated dentin matrix protein 1, bone sialoprotein, or osteopontin results in the protection of that cell from complement-mediated lysis. A variety of pathogenic microbes also express Complement Factor H binding molecules that interfere with immune clearance of the infection.