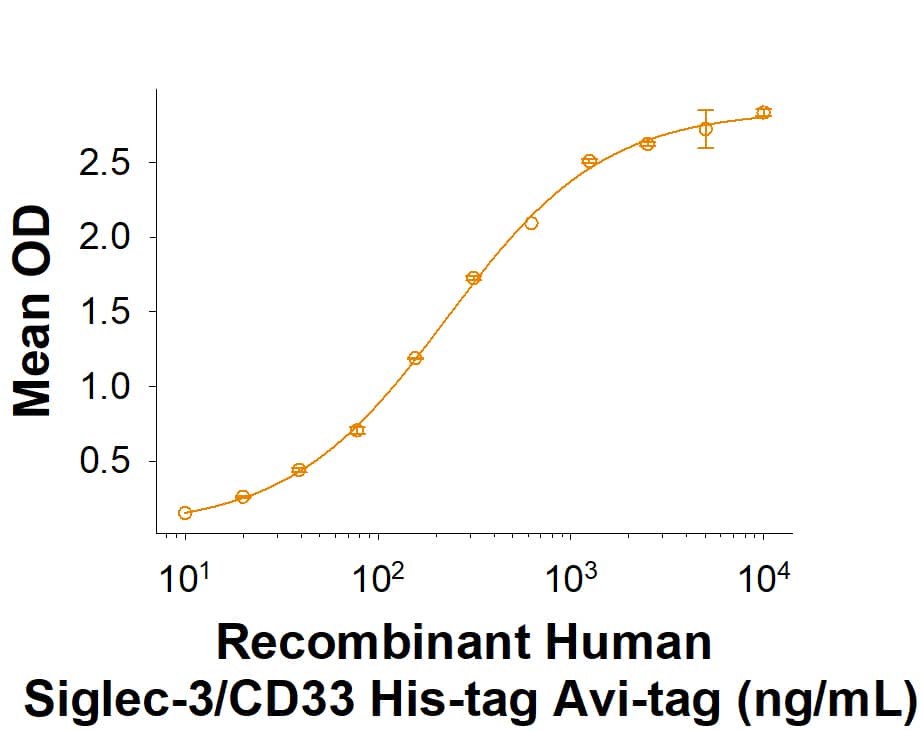
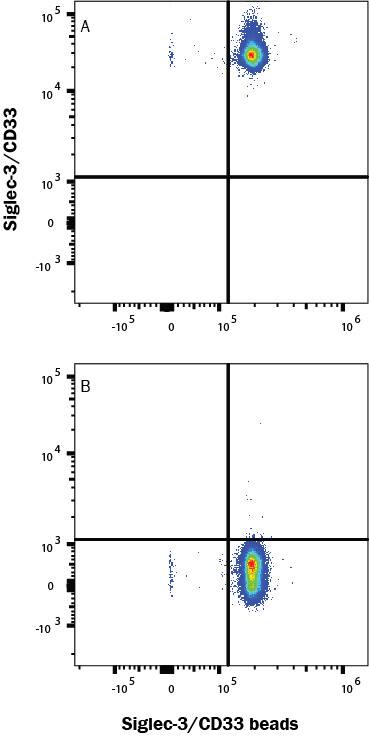
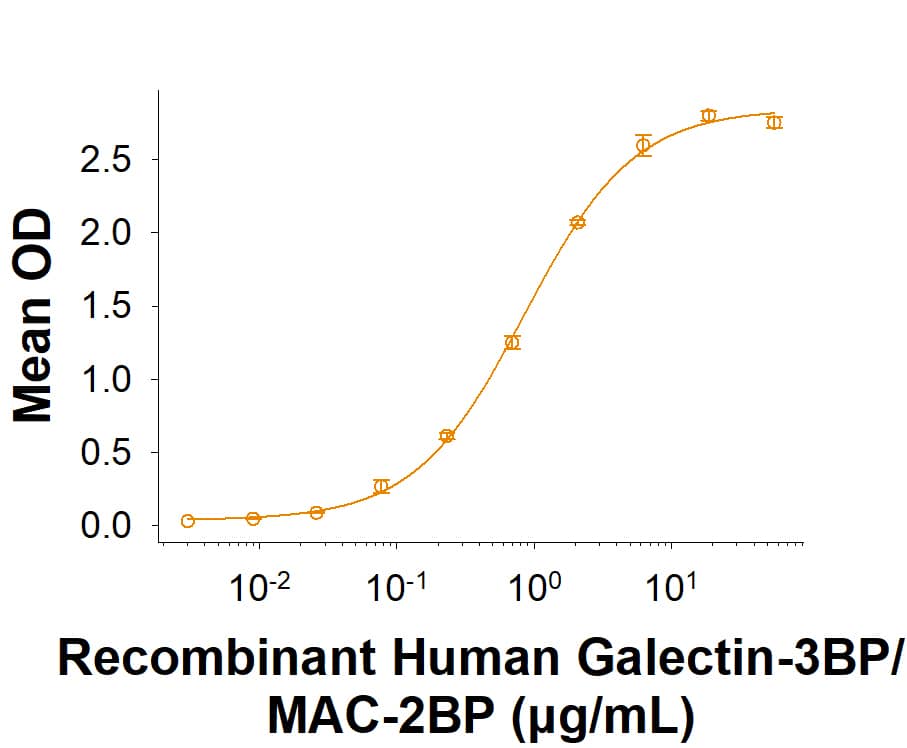
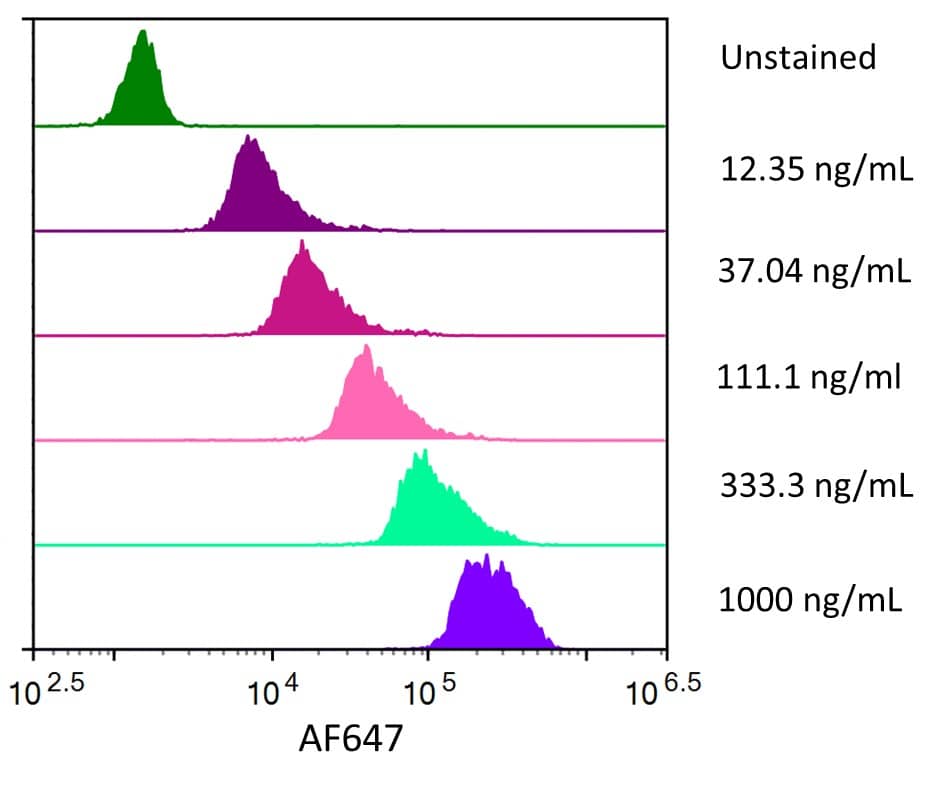
Siglec-3/CD33: Proteins and Enzymes
Siglecs are I-type (Ig-type) lectins belonging to the Ig superfamily. They are characterized by an N-terminal, Ig-like V-type domain that mediates sialic acid binding, followed by varying numbers of Ig-like C2-type domains (2 to 17), a single transmembrane region, and a cytoplasmic tail. The siglecs can be broadly classified into two subgroups: Siglecs-1, -2, and -4, and a Siglec-3/CD33-related subgroup (Siglecs-3, and -5 through -13 in primates) defined by sequence similarity and clustered gene localization. They are widely expressed on hematopoietic cells, often in a cell-type-specific manner, and Siglec-4/MAG is a myelin component in Schwann cells and oligodendrocytes. Their ligands, sialic acids, are negatively charged monosaccharides found on cell-surface glycoproteins and glycolipids. Although Siglec functions continue to be defined, most have intracellular immunoreceptor tyrosine-based inhibitory motifs (ITIM), implicating them in the suppression of immunoreceptor signaling. They may also participate in cell/cell interactions or act as receptors for the entry of viral or bacterial pathogens.
12 results for "Siglec-3/CD33 Proteins and Enzymes" in Products
12 results for "Siglec-3/CD33 Proteins and Enzymes" in Products
Siglec-3/CD33: Proteins and Enzymes
Siglecs are I-type (Ig-type) lectins belonging to the Ig superfamily. They are characterized by an N-terminal, Ig-like V-type domain that mediates sialic acid binding, followed by varying numbers of Ig-like C2-type domains (2 to 17), a single transmembrane region, and a cytoplasmic tail. The siglecs can be broadly classified into two subgroups: Siglecs-1, -2, and -4, and a Siglec-3/CD33-related subgroup (Siglecs-3, and -5 through -13 in primates) defined by sequence similarity and clustered gene localization. They are widely expressed on hematopoietic cells, often in a cell-type-specific manner, and Siglec-4/MAG is a myelin component in Schwann cells and oligodendrocytes. Their ligands, sialic acids, are negatively charged monosaccharides found on cell-surface glycoproteins and glycolipids. Although Siglec functions continue to be defined, most have intracellular immunoreceptor tyrosine-based inhibitory motifs (ITIM), implicating them in the suppression of immunoreceptor signaling. They may also participate in cell/cell interactions or act as receptors for the entry of viral or bacterial pathogens.
| Source: | NS0 |
| Accession #: | AAA51948 |
| Applications: | BA |
Biotinylated
| Source: | CHO |
| Accession #: | P20138.2 |
| Applications: | BA, BA |
Fc Chimera
| Source: | NS0 |
| Accession #: | AAA51948.1 |
| Applications: | BA |
| Source: | CHO |
| Accession #: | P20138.2 |
| Applications: | BA |
| Source: | CHO |
| Accession #: | XP_045235686.1 |
| Applications: | BA |
| Source: | NS0 |
| Accession #: | AAA51948 |
| Applications: | BA |
Fc Chimera
| Source: | NS0 |
| Accession #: | AAA51948.1 |
| Applications: | BA |
| Source: | NS0 |
| Accession #: | AAA51948 |
| Applications: | BA |
| Source: | HEK293 |
| Accession #: | P20138.2 |
| Applications: | BA, BA |
| Source: | NS0 |
| Accession #: | Q63994 |
| Applications: | BA |
| Source: | CHO |
| Accession #: | XP_045235686.1 |
| Applications: | BA |
| Applications: | AC |