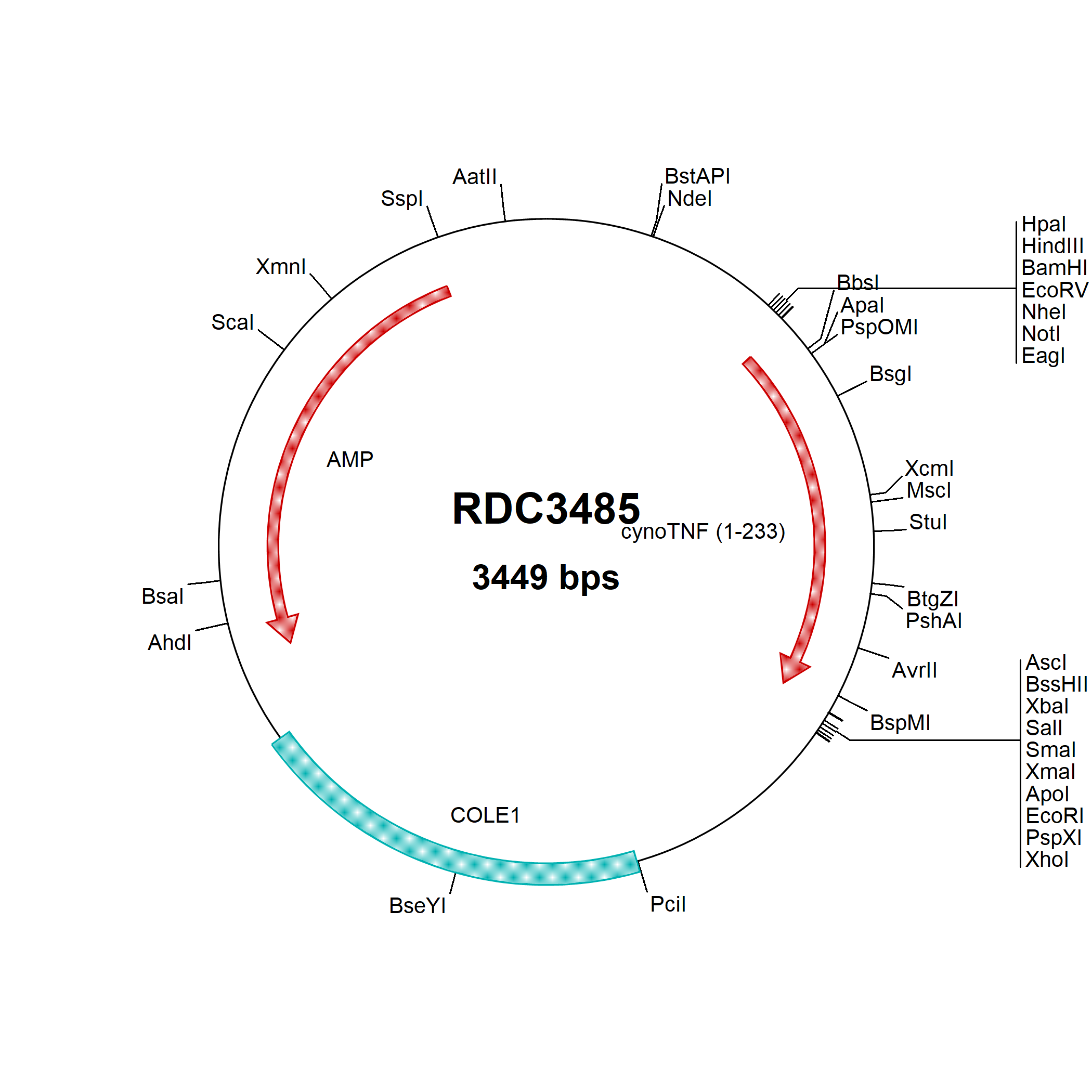
TNF-alpha: cDNA Clones
TNF-alpha (Tumor necrosis factor alpha) plays a central role in inflammation, immune system development, apoptosis, and lipid metabolism. TNF-alpha was first identified as a cytotoxic factor produced by macrophages capable of killing mouse tumor cells. It is the prototypic ligand and along with Lymphotoxin-alpha, were identified as the first members of the TNF superfamily. Active TNF-alpha and other members of the TNF superfamily exist as a homotrimer with high structural homology. Receptor binding occurs at the interface of two TNF-alpha monomers. And receptor activation occurs when all three monomer interfaces are engaged with a receptor. For TNF-alpha, receptor binding and activation occurs through TNF R1 or TNF RII, and subsequently leads to activation of NF-kB or MAPK signaling pathways. Another pathway that TNF-alpha can activate utilizes the death domain of TNF RI to induce apoptosis. TNF-alpha promotes the inflammatory response largely through NF-kB signaling, and inhibition of TNF-alpha has proven successful in treating many autoimmune disorders. TNF-alpha is also present on the cell surface as membrane-bound TNF-alpha can induce the lysis of neighboring tumor cells and virus infected cells. TNF-alpha protein is translated as a type II transmembrane protein containing an N-terminal transmembrane domain. The soluble cytokine is released from its cell-anchoring TM domain by proteolytic processing by metalloproteases.
4 results for "TNF-alpha cDNA Clones" in Products
4 results for "TNF-alpha cDNA Clones" in Products
TNF-alpha: cDNA Clones
TNF-alpha (Tumor necrosis factor alpha) plays a central role in inflammation, immune system development, apoptosis, and lipid metabolism. TNF-alpha was first identified as a cytotoxic factor produced by macrophages capable of killing mouse tumor cells. It is the prototypic ligand and along with Lymphotoxin-alpha, were identified as the first members of the TNF superfamily. Active TNF-alpha and other members of the TNF superfamily exist as a homotrimer with high structural homology. Receptor binding occurs at the interface of two TNF-alpha monomers. And receptor activation occurs when all three monomer interfaces are engaged with a receptor. For TNF-alpha, receptor binding and activation occurs through TNF R1 or TNF RII, and subsequently leads to activation of NF-kB or MAPK signaling pathways. Another pathway that TNF-alpha can activate utilizes the death domain of TNF RI to induce apoptosis. TNF-alpha promotes the inflammatory response largely through NF-kB signaling, and inhibition of TNF-alpha has proven successful in treating many autoimmune disorders. TNF-alpha is also present on the cell surface as membrane-bound TNF-alpha can induce the lysis of neighboring tumor cells and virus infected cells. TNF-alpha protein is translated as a type II transmembrane protein containing an N-terminal transmembrane domain. The soluble cytokine is released from its cell-anchoring TM domain by proteolytic processing by metalloproteases.