Learn More About Controls for ICC/IF Experiments
Controls for ICC/IF Experiments | What is Autofluorescence and Why is it Important in ICC/IF?
Controls for ICC/IF Experiments
There are several variables in ICC/IF which can contribute to background staining and false-positive results. To identify potential sources of non-specific staining, the appropriate controls should be included in each experiment. Listed below are some of the controls which are essential for ICC/IF experiments.
Autofluorescence control
Autofluorescence is a naturally occurring or fixation-induced fluorescence that can contribute to false positive staining and bleed-through artifacts. Prior to staining, cells (fixed, blocked, permeabilized, but not stained) should be examined for fluorescence emissions using a fluorescence microscope with the appropriate filter sets.
No primary control
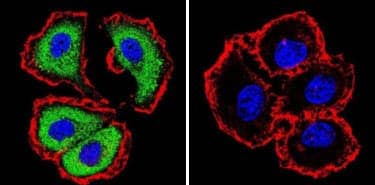
A no primary control refers to a sample that is not probed with the primary antibody but is subjected to equivalent assays conditions (e.g. fixed, blocked, permeabilized and incubated with secondary antibody). This control helps determine the specificity of the primary antibody. The specificity of an NFATc1 antibody is shown in the example below.
+ Primary / + Secondary
CC/IF analysis of NFATc1 in MCF7 cells with (or without) NFATc1 antibody (7A6) [NB300-620] and using DyLightTM 488 conjugated secondary antibody (green). Phalloidin (red) and DAPI (blue) were used for counterstaining the cytoskeleton and nuclei, respectively. Note that NFATc1 staining is negligible for fixed cells that were not probed with the NFATc1 antibody.
Peptide block (aka absorption control)
Blocking an antibody with a peptide or protein, often the immunogen used for antibody production, is recommended to confirm antibody specificity. For this control, the primary antibody is first inactivated by preincubation with the immunogen and then used in place of the primary antibody under equivalent assay conditions. The absence of staining with this control indicates the primary antibody specifically recognizes and binds the target antigen. Learn more about Peptide Blocking.
Isotype control
Isotype controls refer to immunoglobulin molecules that are raised in the same species as the primary antibody and have the same isotype (e.g., IgG, IgG1, IgG2a, IgM etc.) but do not specifically target the antigen. Used in place of the primary antibody, this control helps determine if non-specific background staining is caused by the primary antibody binding to Fc receptors. Learn more about Isotype Controls.
Expression controls
Negative controls include cells with known siRNA/shRNA gene-mediated silencing, or cells derived from knockout models that lack or have reduced expression of the target protein. Examples include HIF1 alpha knock-out MEFs as a negative control for HIF1 alpha, and MMP7 null cell line SW480 as a negative control for MMP7.
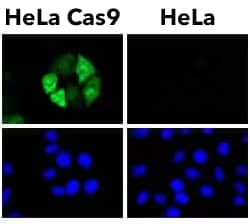
Positive controls include cells with known or exogenous expression of the target protein e.g. CRISPR-Cas9 transfected HeLa cells as a positive control for CRISPR-Cas9 (see example below), and chloroquine treated HeLa cells as a positive control for LC3B.
CRISPR-Cas9 was only detected in HeLa cells expressing Flag-tagged SpyCas9 using CRISPR-Cas9 antibody (clone 6G12) [NBP2-52398] with Alexa Fluor® 488 conjugated secondary antibody. Nuclei were counterstained with Hoechst 33342.
What is Autofluorescence and Why is it Important in ICC/IF?
Autofluorescence can contribute to background signal and result in false-positive staining. It is important to observe cells under a microscope before immunostaining to determine if autofluorescence is present in your samples. Based on its origin, autofluorescence is classified into two major types: (i) biological/inherent, and (ii) fixative-induced autofluorescence as discussed below:
| Biological/inherent autofluorescence Cells or tissues often contain biological components which emit fluorescence signal when excited by a suitable wavelength. Biological autofluorescence originates from mitochondria, lysosomes, and aromatic amino acid containing components including flavin coenzymes (FAD, FMN) and pyridine nucleotides (NADH). Below is an example of autofluorescence emitted by Lipofuscin, a pigment that accumulates with age in many tissue types. Lipofuscin has autofluorescence properties that overlap with the emission spectra of commonly used fluorochromes |
Fixation induced autofluorescence In samples fixed with aldehydes, such as formaldehyde, the aldehyde group can react with amine groups of proteins, resulting in end products which exhibit fluorescent properties. Aldehyde fixative- induced autofluorescence can be blocked by reducing the aldehyde group to a hydroxyl group, or by treating fixed cells with sodium borohydride. Quencher treatment time should be optimized in order to quench autofluorescence while minimizing the impact on specific immunostaining. |
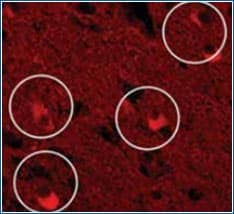
Example of Biological Autofluorescence
Circled in the micrograph are Lipofuscin-containing neurons that may appear labeled using fluorescence microscopy in the red spectrum.
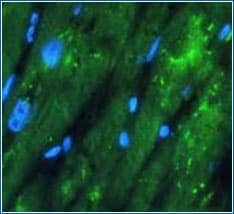
Example of Fixative-induced Autofluorescence
Formalin-fixed sample showing strong autofluorescence (green) in human cardiomyocytes. The blue color represents nuclear staining with DAPI.