Recovery and Linearity Are Key Tests of Immunoassay Accuracy
R&D Systems, a Bio-Techne brand, has decades of immunoassay development and manufacturing experience. Our expertise is reflected in the consistently high quality of our industry-leading immunoassays. Here we compare our Type 1 Interferon QuantikineTM ELISA kits to the competition. The competition fails to meet commonly accepted standards for recovery and linearity. We also show how easy it is to move from one Bio-Techne immunoassay platform to the next.
Type 1 IFN Quantikine ELISA Kits Have Excellent Recovery
Recovery or spike recovery tests are used to evaluate assay accuracy. Immunoassays should recover 80-120% of the spiked target protein. Failure to recover at least 80% of the spiked protein indicates that something in the sample is interfering with the assay and that it may return inaccurate data.
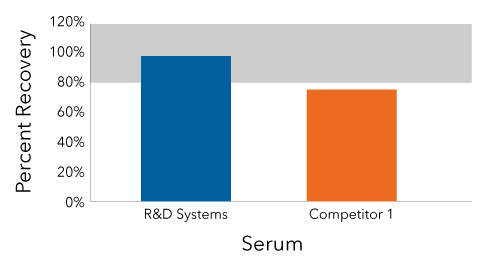
The Competitor ELISA Failed to Demonstrate Adequate IFN Alpha Recovery
In this experiment, human serum was spiked with recombinant IFN alpha. IFN alpha was quantified using the Human IFN Alpha All-Subtype Quantikine ELISA Kit (Catalog # DFNAS0) and a competitor ELISA. Only the Quantikine ELISA recovered within 80-120% of the spiked concentration. The competitor ELISA "under-recovered" IFN alpha, indicating that it is susceptible to sample matrix effects.
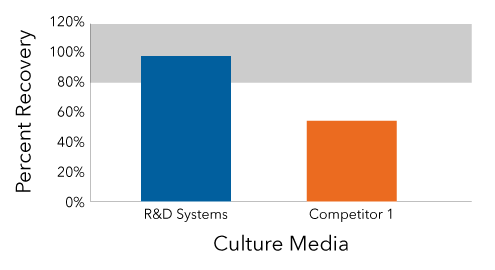
Only the Quantikine ELISA Kit Demonstrated Adequate IFN Beta Recovery
In this experiment, cell culture media was spiked with recombinant IFN beta. IFN beta was quantified using the Human IFN Beta Quantikine ELISA Kit (Catalog # DIFNB0) and competitor ELISA Kit. Only the Quantikine ELISA had a recovery between 80% and 120%.
Type 1 IFN Quantikine ELISA Kits Have Excellent Linearity of Dilution
Linearity of dilution evaluates immunoassay accuracy and consistency. Immunoassays should recover 80-120% of the target analyte from each sample of a dilution series when back-calculated. If the percent recovery increases as a function of sample dilution, it indicates that interfering substances are being diluted out. Immunoassays that fail this test increase the likelihood of repeat experiments due to inconsistent data.
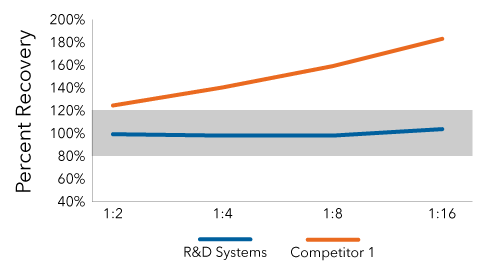
The Competitor IFN Alpha ELISA Did Not Maintain Linearity of Dilution
Conditioned media from Polyinosinic:polycytidylic acid (poly (I:C)) treated cells was serially diluted and IFN alpha was quantified using the Mouse IFN Alpha Quantikine ELISA Kit (Catalog # MFNAS0) and the competitor ELISA. The competitor ELISA failed to maintain linearity of dilution within the acceptable range of 80%-120%.
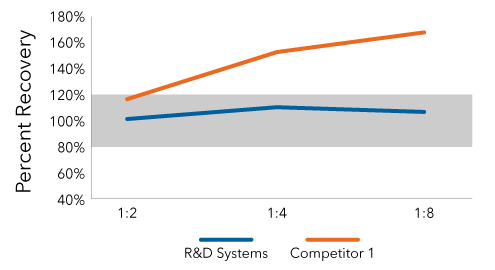
The Human IFN Beta Quantikine ELISA Kit Maintained Natural Linearity
Conditioned media from Polyinosinic:polycytidylic acid (poly (I:C)) treated cells was serially diluted and IFN beta was quantified using the Human IFN Beta Quantikine ELISA Kit and a competitor ELISA. The Quantikine ELISA maintained linearity of dilution between 80% and 120%. The competitor ELISA failed to do so.
Move Seamlessly From Discovery to Validation
Move seamlessly from Luminex® multiplex assays to Quantikine ELISA Kits or Simple Plex automated immunoassays. Quantify up to 50 analytes in a sample during early-stage discovery work with Luminex Assays. Validate your key target analytes with gold standard Quantikine ELISA Kits or in 90 minutes with Simple Plex assays.
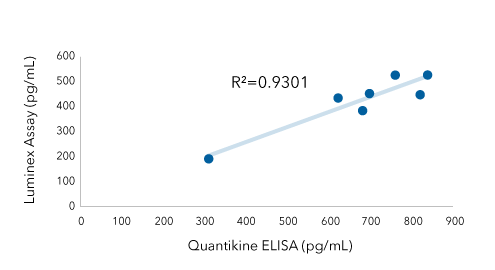
IFN Alpha: Luminex® Assay Vs Quantikine ELISA Kit
IFN alpha was quantified in conditioned media from Polyinosinic: polycytidylic acid (poly (I:C)) treated cells using the Human IFN Alpha All Subtype Quantikine ELISA Kit (Catalog # DFNAS0) and the IFN Alpha Luminex Assay. Data from the two assays are highly correlated (R2=0.9301), indicating that you can move from one assay to another and maintain consistent data.
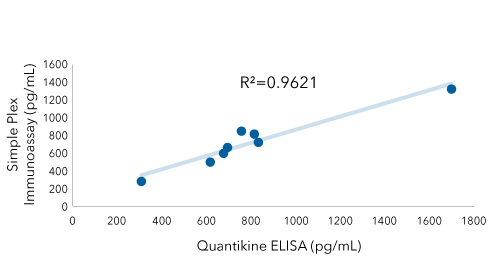
IFN Alpha: Quantikine ELISA Kit Vs Simple Plex Assay
IFN alpha was quantified in conditioned media from Polyinosinic: polycytidylic acid (poly (I:C)) treated cells using the Human IFN Alpha All Subtype Quantikine ELISA Kit (Catalog # DFNAS0) and the Human IFN Alpha Multi-Subtype Simple Plex Assay (Catalog # SPCKB-PS-002812). The data from the two assays are highly correlated (R2=.9621) indicating that you can move seamlessly between Bio-Techne’s immunoassay solutions and maintain consistent results.
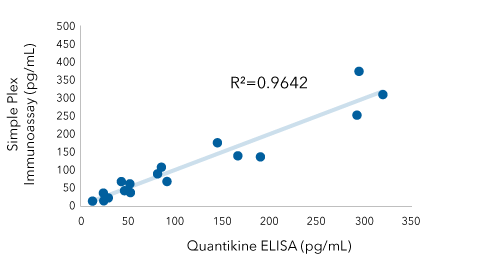
IFN Beta: Quantikine ELISA Kit Vs Simple Plex Assay
IFN beta was quantified in cell culture media with and without serum or plasma spikes using the Human IFN Beta Quantikine ELISA Kit (Catalog # DIFNB0) and the Simple Plex IFN Beta immunoassay (Catalog # SPCKB-PS-000934) A robust correlation (R2=0.9642) exists between data generated on both platforms indicating that you can easily move from one platform to the next.
Choose The Type 1 Interferon Immunoassay That Best Suits Your Needs
Luminex® Immunoassays
Luminex® Immunoassays
Luminex Assays save time and precious samples. Quantify up to 50 analytes per sample. Choose from 450 targets for Luminex Discovery assays.
Quantikine ELISA Kits
Quantikine ELISA Kits
Quantikine ELISA Kits are the most referenced on the market. Count on Quantikine ELISA Kits for reliable, consistent performance over the long term.

Simple Plex™ Immunoassays
Simple Plex™ Immunoassays
Simple Plex automated immunoassays minimize hands-on time and user error. Get highly reproducible data in 90 minutes.
Purchase IFN Alpha Simple Plex Assays
Resources for Interferon Research
In 1957 Isaacs and Lindenmann coined the term “Interferon” to describe the “non-haemagglutinating macromolecular particle” responsible for viral interference (1). At present, are 3 major classes of interferons (IFNs), Type I, Type II, and Type III. Interferon alpha (IFN-α), along with IFN-β, IFN-δ, IFN-ε, IFN-κ, IFN-ώ, and IFN-τ are all Type I IFNs (2). The sole type II IFN is IFN-γ. Type III IFNs include IFN-λ1, IFN-λ2, IFN-λ3 and IFN-λ4(3). Type I IFNs, are induced in response to viral nucleic acids such as double-stranded DNA or RNA (dsDNA, dsRNA) and single-stranded RNA (ssRNA), viral glycoproteins, microbial cytosine-phosphate-guanosine (CpG) DNA, DNA damage, and chromosomal instability (4,5).
- Isaacs, A. and J Lindenmann (1957) Proc. of the Royal Soc. B. 147: 258
- Murira, A. & A. Lamarre (2016) Front. Immunol. 7:1
- Lazear, H. M. et al (2019) Immunity. 50:909
- Tomasello, E. et al, (2014) Front. Immunol. 5:1
- Fuertes, M.B. et al (2013) Trends Immunol. 34:67
Luminex® is a Registered Trademark of Luminex Corporation.



