Immunohistochemical Staining of Histone H2AX in Tumors from Treated Mice
Immunocompromised mice were subcutaneously injected with cancerous cell lines and tumors were allowed to establish. Treatments occurred every other day and the studied compound or the equivalent vehicle control administered intraperitoneally for five weeks. Tumor volume and mass were measured two times per week. IHC analysis of sectioned tumor tissues from the MDA-MB-231 study. Each section was subjected to the specified antibody followed by a biotinylated secondary antibody. Detection was done using a DAB Peroxidase HRP Substrate Kit (brown) followed by Hematoxylin counterstaining (purple). Images were obtained using inverted bright field microscopy. Sectioning results are representative of three individual tumors. Scale bar is 50 microns. Image collected and cropped by CiteAb from the following publication (nature.com/articles/s41598-017-01230-4), licensed under a CC-BY license.
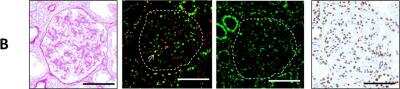
Immunohistochemical Staining of Histone H2AX in Human Kidney Tissue
Immunostaining of Histone H2AX [p Ser139] WT1 and 5mC in patients with IgA nephropathy and controls. Examples of PAS staining and immunostaining with Histone H2AX [p Ser139] (green) and WT1 (red), pATM and 5mC in glomeruli of IgA nephropathy and controls. A kidney sample of a 65-year-old male of IgA nephropathy without podocytopathic features. Arrows indicate Histone H2AX [p Ser139] and WT1 double-positive cells. Scale bars: 50um. Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/s41598-019-57140-0) licensed under a CC-BY license.
Western Blot Detection of Histone H2AX in EPE Treated HeLa Cells
Samples: Nuclear extract from HeLa cells treated with 100 uM EPE for 4 hours (+) or mock treated (-). Antibody: Affinity purified rabbit Histone H2AX [p Ser139] antibody used at 0.1 ug/ml. Detection: Chemiluminescence with an exposure time of 3 minutes. Band appears at an observed molecular weight of ~17 kDa.
Western Blot Detection of Histone H2AX in EPE Treated Jurkat Cells
Detection of human Histone H2AX [p Ser139] by western blot. Samples: Whole cell lysate (50 ug) from Jurkat cells treated with 100 uM EPE for 4 hours (+) or mock treated (-). Antibody: Affinity purified rabbit Histone H2AX [p Ser139] antibody NB100-384 used for WB at 0.1 ug/ml. Detection: Chemiluminescence with an exposure time of 3 seconds. Band appears at an observed molecular weight of ~18 kDa.
Immunohistochemical Analysis of Histone H2AX in Paraffin Embedded Mouse CT26 Colon Carcinoma
FFPE section of mouse CT26 colon carcinoma. Antibody: Affinity purified rabbi Histone H2AX [p Ser139] antibody used at a dilution of 1:1,000 (1 ug/ml). Detection: DAB.
Flow Cytometry of EPE Treated Jurkat Cells Stained with Histone H2AX Antibody
Analysis of Histone H2AX [p Ser139] in EPE Treated Jurkat Cells. Cells were treated for 3 hrs in 5ug/ml etoposide, fixed in 1.5% PFA, and permeabilized in 90% Methanol. 1 million cells were stained with 0.5 ug anti-KLH or anti-H2AX NB100-384 and secondary FITC-conjugated goat anti-rabbit (in a 150ul reaction). Black- etosposide treated, anti-KLH; Red- untreated, anti-Histone H2AX [p Ser139]; Blue- etoposide treated , anti-Histone H2AX [p Ser139].
Immunohistochemical Detection of Histone H2AX in Paraffin Embedded Human Ovarian Carcinoma
FFPE section of human ovarian carcinoma. Antibody: Affinity purified rabbit Histone H2AX [p Ser139] antibody used at a dilution of 1:5,000 (0.2 ug/ml). Detection: DAB.
Simple Western Analysis of Histone H2AX in Jurkat Cell Lysate
Electropherogram image(s) of corresponding Simple Western lane view. Histone H2AX [p Ser139] antibody was used at 5 ug/ml dilution on Jurkat lysate(s).
Immunohistochemistry: Rabbit Polyclonal Histone H2AX [p Ser139] Antibody -
Immunohistochemistry: Rabbit Polyclonal Histone H2AX [p Ser139] Antibody - Histone H2AX Antibody on mouse cancer tissue. H2AX (Gray) and H2BGFP(Green). Primary antibody dilution: 1:1000 in a 10um slice.
Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] -
mTORC1/2 activity prevents Cisplatin-induced cell death in MCF-10A cells. (A) Western blot displaying effects on mTOR signaling during a dose escalation of PP242 treatment in MCF-10A cells; (B) Western blot displaying effects of mTOR signaling on a dose escalation of cisplatin treatment in MCF-10A cells; (C) Western blot displaying effects on mTOR signaling and cell death during non-treated, Cisplatin, PP242, and Cisplatin + PP242-treated MCF-10A cells.
Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] -
Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] - ATM & NF-kappa B activation are downregulated in Ercc1-/ delta mice heterozygous for Atm. (A) Livers were collected at 12 weeks of age from WT, Ercc1-/ delta & Ercc1-/ deltaAtm+/- mic (n=3 per genotype) & lysates analyzed by western blot for activation of ATM & its downstream effectors. (B) Same liver lysates were used to measure phosphorylation of p65 & I kappaB alpha. (C) Western blot analysis of livers from 16-week-old WT, Ercc1-/ delta & Ercc1-/ deltaAtm+/-mice (n=3 per genotype) probed for activation of ATM. GAPDH was used as a loading control. (D) Same liver lysates used to measure activation of NF-kappa B. (E) Fourteen-week-old livers from Ercc1-/ delta & Ercc1-/ deltap65+/- mice (n=3 per genotype) were analyzed by western blot for activation of ATM (F) & NF-kappa B. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32201398), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunocytochemistry/ Immunofluorescence: Histone H2AX [p Ser139] Antibody [NB100-384] -
Immunocytochemistry/ Immunofluorescence: Histone H2AX [p Ser139] Antibody [NB100-384] - Immunostaining of gammaH2AX, WT1 & 5mC in patients with IgA nephropathy & controls. Examples of PAS staining & immunostaining with gammaH2AX (green) & WT1 (red), pATM & 5mC in glomeruli of IgA nephropathy & controls. (A) A control kidney sample of 44-year-old female, (B) 65-year-old male of IgA nephropathy without podocytopathic features & (C) 55-year-old male of IgA nephropathy with podocytopathic features. Arrows indicate gammaH2AX & WT1 double-positive cells. Scale bars: 50 μm. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31937846), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunocytochemistry/ Immunofluorescence: Histone H2AX [p Ser139] Antibody [NB100-384] -
Immunocytochemistry/ Immunofluorescence: Histone H2AX [p Ser139] Antibody [NB100-384] - UHRF1 deficiency resulted in impaired meiotic recombination & defective pachynema.a Double immunofluorescence of SYCP3 (green) & DMC1 (red) in testicular spread preparations. b, c The number of DMC1 foci in zygotene stage (b) & pachytene stage (c). d Immunostaining for SYCP3 (red) & gammaH2AX (green). e The percentage of abnormal gammaH2AX foci in the pachytene stage. f Immunostaining for SYCP3 (red) & MLH1 (green). g The number of MLH1 foci in pachynema. h Immunostaining for SYCP3 (red) & H1t (green). i The percentage of spermatocytes with H1T staining. ***p ≤ 0.001; *p ≤ 0.05. Scale bar, 5 μm in a, d, f, h. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32081844), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] -
Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] - mTORC1/2 activity prevents Cisplatin-induced cell death in MCF-10A cells. (A) Western blot displaying effects on mTOR signaling during a dose escalation of PP242 treatment in MCF-10A cells; (B) Western blot displaying effects of mTOR signaling on a dose escalation of cisplatin treatment in MCF-10A cells; (C) Western blot displaying effects on mTOR signaling & cell death during non-treated, Cisplatin, PP242, & Cisplatin + PP242-treated MCF-10A cells. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31771139), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunocytochemistry/ Immunofluorescence: Histone H2AX [p Ser139] Antibody [NB100-384] -
Immunocytochemistry/ Immunofluorescence: Histone H2AX [p Ser139] Antibody [NB100-384] - Immunostaining of gammaH2AX, WT1 & 5mC in patients with IgA nephropathy & controls. Examples of PAS staining & immunostaining with gammaH2AX (green) & WT1 (red), pATM & 5mC in glomeruli of IgA nephropathy & controls. (A) A control kidney sample of 44-year-old female, (B) 65-year-old male of IgA nephropathy without podocytopathic features & (C) 55-year-old male of IgA nephropathy with podocytopathic features. Arrows indicate gammaH2AX & WT1 double-positive cells. Scale bars: 50 μm. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31937846), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunocytochemistry/ Immunofluorescence: Histone H2AX [p Ser139] Antibody [NB100-384] -
Immunocytochemistry/ Immunofluorescence: Histone H2AX [p Ser139] Antibody [NB100-384] - Immunostaining of gammaH2AX, WT1 & 5mC in patients with IgA nephropathy & controls. Examples of PAS staining & immunostaining with gammaH2AX (green) & WT1 (red), pATM & 5mC in glomeruli of IgA nephropathy & controls. (A) A control kidney sample of 44-year-old female, (B) 65-year-old male of IgA nephropathy without podocytopathic features & (C) 55-year-old male of IgA nephropathy with podocytopathic features. Arrows indicate gammaH2AX & WT1 double-positive cells. Scale bars: 50 μm. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31937846), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] -
Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] - ATM & NF-kappa B activation are downregulated in Ercc1-/ delta mice heterozygous for Atm. (A) Livers were collected at 12 weeks of age from WT, Ercc1-/ delta & Ercc1-/ deltaAtm+/- mic (n=3 per genotype) & lysates analyzed by western blot for activation of ATM & its downstream effectors. (B) Same liver lysates were used to measure phosphorylation of p65 & I kappaB alpha. (C) Western blot analysis of livers from 16-week-old WT, Ercc1-/ delta & Ercc1-/ deltaAtm+/-mice (n=3 per genotype) probed for activation of ATM. GAPDH was used as a loading control. (D) Same liver lysates used to measure activation of NF-kappa B. (E) Fourteen-week-old livers from Ercc1-/ delta & Ercc1-/ deltap65+/- mice (n=3 per genotype) were analyzed by western blot for activation of ATM (F) & NF-kappa B. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32201398), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] -
Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] - Pharmacologic inhibition of ATM rescues oxidative stress-induced senescence by suppressing ATM- & NEMO-mediated NF-kappa B activation. (A) Representative images of primary WT & Ercc1-/- MEFs were induced to undergo senescence by serial passaging at 20% oxygen. At passage 5, MEFs were grown in the presence or absence of KU-55933 (10 μM) for 72 hrs. Senescence was determined by SA-beta gal staining. Images were obtained at the magnification of 10x. (B) Quantitation of the percent SA-beta gal positive cells. Graph represents the mean +/- s.e.m. of three independent experiments. Student’s t-test, ***p <0.001, ****p <0.0001. (C) Passage 5 Ercc1-/- MEFs treated with vehicle or KU-55933 (10 μM) for 72 hours were collected & levels of p21 & p16INK4a were determined by western blotting. (D) Passage 5 Ercc1-/- MEFs were treated with KU-55933 (10 μM) for 72 hours & whole cell lysate (CL) & nuclear extracts (NE) were analyzed by immunoblotting for expression of proteins involved in the DNA damage response. (E) Whole cell lysate (CL) & nuclear extract (NE) were extracted from Ercc1-/- MEFs treated with 10 μM of KU-55933 for analysis of nuclear NEMO & p65. GAPDH was used as a loading control of total proteins & LaminA/C as a loading control of nuclear protein. (F) Passage 5 WT & Ercc1-/- MEFs transfected with a NF-kappa B-luciferase reporter construct were cultured in the presence or absence of KU-55933 (10 μM) & were collected for luciferase assays after 72 hours. (G) qRT-PCR analysis of mRNA expression in passage 5 WT & Ercc1-/- MEFs treated with or without of KU-55933 (10 μM) for 72 hrs. P values were determined using a Student’s t-test. *p<0.05, **p<0.01, ***p <0.001. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32201398), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunohistochemistry: Histone H2AX [p Ser139] Antibody [NB100-384] -
Immunohistochemistry: Histone H2AX [p Ser139] Antibody [NB100-384] - Orally administered lemongrass & white tea extract reduce tumor size in lymphoma xenograft model in immunocompromised miceImmunocompromised mice were subcutaneously injected with cancerous cells & tumors were allowed to establish. Treatments occurred every other day & the studied compound or the equivalent vehicle control administered orally for three weeks. (A, B) The tumors were photographed before & after extraction from the animals. (C) Tumor volume & mass were measured two times per week. (D) Immunohistochemistry analysis of sectioned tumor tissues from the lymphoma study. Each section was subjected to the specified antibody followed by a biotinylated secondary antibody. Detection was done using a DAB Peroxidase HRP Substrate Kit (brown) followed by Hematoxylin counterstaining (purple). Images were obtained using inverted bright field microscopy. Sectioning results are representative of three individual tumors. Scale bar is 50 microns. Statistical analysis using One-Way ANOVA. *p < 0.05 vs tumor volume of the control. Image collected & cropped by CiteAb from the following publication (https://www.oncotarget.com/lookup/doi/10.18632/oncotarget.22502), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] -
Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] - CPT induces transcription- & proteasome-dependent apoptosis in quiescent WI38 hTERT cells. (A & B) Serum-starved cells were treated with DMSO or FLV (1 μM) for 1 h before the addition of DMSO or CPT (25 μM) for 16 & 24 h. (A) Percentages of cells that remained attached to culture flasks (means ± SD of quadruplicates). (B) Cell survival was analyzed by a CellTiter-Blue assay (means ± SD of triplicates). (C–G) Western blot of the indicated proteins in serum-starved cells treated for 1 h with DMSO or with FLV (1 μM) (C), lactacystin (10 μM) (D), veliparib (5 μM) or olaparib (10 μM) (E), ATMi (10 μM) (F) or DNA-PKi (10 μM) (G) before the addition of DMSO (‘-’, 24 h in panels C & F) or CPT (25 μM) for the indicated times. Data shown are representatives from two to three experiments. PARPCL: cleaved PARP. H2AX & alphaTubulin were the loading controls. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26578593), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] -
Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] - Inhibition of DNA-PK prevents monoubiquitination of H2AX & H2A in CPT-treated quiescent WI38 hTERT cells. (A–E) Serum-starved cells were treated with DMSO or DNA-PKi (10 μM) for 1 h before the addition of DMSO (untreated) or CPT (25 μM) for 1 h. (A) Western blot of gammaH2AX. +Ub1 indicates gammaH2AX monoubiquitinated. The top panel shows quantification of Ub1-gamma H2AX normalized to alphaTubulin (means ± SEM, n = 4). **P < 0.01. (B & C) Cells were pre-extracted with CSK buffer before co-staining for Ub-H2A (red) & 53BP1 phosphorylated on S1778 (p53BP1) (green). (B) Representative pictures. Images were merged to determine colocalization (yellow). The large Ub-H2AX focus at the periphery of the nuclei of untreated & CPT-treated cells may marks the inactive X chromosome as reported (91). (C) Percentages of nuclei with at least 5 Ub-H2A foci (means ± SEM, n = 3, 100 nuclei were analyzed for each treatment in each experiment). ***P < 0.001. (D & E) Cells were co-stained for ubiquitinated proteins (FK2, red) & gammaH2AX (green). (D) Representative pictures. Images were merged to determine colocalization (yellow). (E) Number of FK2 foci per nucleus from one representative experiment (76–111 nuclei were analyzed for each treatment) out of three. ****P < 0.0001. In the microscopic images, nuclear contours, identified by DAPI staining (blue in the merge images at bottom), are indicated by dashed lines. Bars: 10 μm. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26578593), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunocytochemistry/ Immunofluorescence: Histone H2AX [p Ser139] Antibody [NB100-384] -
Immunocytochemistry/ Immunofluorescence: Histone H2AX [p Ser139] Antibody [NB100-384] - The production of DSBs depends on Top1 degradation in CPT-treated quiescent cells. (A–C) Serum-starved WI38 hTERT cells were co-transfected with siRNAs against cullin 3 & cullin 4B or against a control sequence & then treated with DMSO (−CPT) or 25 μM CPT (+CPT) for 1 h. (A & B) Western blotting of the indicated proteins. alphaTubulin: loading control. (C) Number of gammaH2AX foci per nucleus from one representative experiment (246–348 nuclei were analyzed for each treatment) out of three. ***P < 0.001. (D & E) Serum-starved WI38 hTERT cells were treated with DMSO or MG132 (50 μM) for 1 h before exposure to 0.8 Gy IR. One hour post-irradiation, cells were co-stained for gammaH2AX (green) & 53BP1 (red). (D) Representative pictures. (E) Number of gammaH2AX foci per nucleus from one representative experiment (162–180 nuclei were analyzed for each treatment) out of three. Ns: not significant. (F & G) U2OS EV28 cells were treated with DMSO or MG132 (10 μM) for 1 h before the addition of ethanol (untreated) or 300 nM 4-hydroxitamoxifen (4OHT) for 4 h to express AsiSI in the nucleus (42). (F) Representative pictures of cells co-stained for gammaH2AX (green) & 53BP1 (red). (G) ChIP analysis using an anti-gamma H2AX antibody (black) or a non-immune antibody (IgG, gray). Enrichment was assessed by QPCR amplification using primers proximal to two AsiSI sites located inside two genes (Gene I: SFRS6, Gene II: CCD47) & primers distal to an AsiSI site (Control). Enrichment was normalized to the maximum recovery for each experiment (means ± SEM, n = 3). Ns: not significant; *P < 0.05. In the microscopic images, nuclear contours, identified by DAPI staining (not shown), are indicated by dashed lines. Bars: 10 μm. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26578593), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunocytochemistry/ Immunofluorescence: Histone H2AX [p Ser139] Antibody [NB100-384] -
Immunocytochemistry/ Immunofluorescence: Histone H2AX [p Ser139] Antibody [NB100-384] - Inhibition of DNA-PK prevents monoubiquitination of H2AX & H2A in CPT-treated quiescent WI38 hTERT cells. (A–E) Serum-starved cells were treated with DMSO or DNA-PKi (10 μM) for 1 h before the addition of DMSO (untreated) or CPT (25 μM) for 1 h. (A) Western blot of gammaH2AX. +Ub1 indicates gammaH2AX monoubiquitinated. The top panel shows quantification of Ub1-gamma H2AX normalized to alphaTubulin (means ± SEM, n = 4). **P < 0.01. (B & C) Cells were pre-extracted with CSK buffer before co-staining for Ub-H2A (red) & 53BP1 phosphorylated on S1778 (p53BP1) (green). (B) Representative pictures. Images were merged to determine colocalization (yellow). The large Ub-H2AX focus at the periphery of the nuclei of untreated & CPT-treated cells may marks the inactive X chromosome as reported (91). (C) Percentages of nuclei with at least 5 Ub-H2A foci (means ± SEM, n = 3, 100 nuclei were analyzed for each treatment in each experiment). ***P < 0.001. (D & E) Cells were co-stained for ubiquitinated proteins (FK2, red) & gammaH2AX (green). (D) Representative pictures. Images were merged to determine colocalization (yellow). (E) Number of FK2 foci per nucleus from one representative experiment (76–111 nuclei were analyzed for each treatment) out of three. ****P < 0.0001. In the microscopic images, nuclear contours, identified by DAPI staining (blue in the merge images at bottom), are indicated by dashed lines. Bars: 10 μm. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26578593), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] -
Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] - DDR & NF-kappa B are activated concomitantly in senescent MEFs & aged tissues. (A) Immunoblot detection of p-p65 & total p65 in liver tissue from 16-week-old WT (n=3) & Ercc1-/ delta (n=3) mice. (B) Immunoblot detection of phosphorylation of ATM & downstream targets gammaH2AX & p21 in liver from 16-week-old WT & Ercc1-/ delta mice. (C) Immunoblot detection of phosphorylation of NF-kappa B & I kappaB alpha in liver lysates from 3, 12 & 24 month-old WT mice. n=3 mice per group. (D) Immunoblot detection of p-ATM, ATM & p21 in the same liver lysates. (E) Immunoblot detection of DDR effectors in nuclear extracts from passage 5 WT & Ercc1-/- MEFs, grown at 20% oxygen. (F) Level of NF-kappa B activation is higher in Ercc1-/- MEFs compared to WT MEFs at passage 5, as measured by Immunoblot detection of p-p65 & total p65 in WT & Ercc1-/- MEFs at passage 5 after culturing in 20% oxygen. (G) Representative images of immunofluorescent detection of p65 & NEMO in passage 4 WT & Ercc1-/- MEFs grown at 20% oxygen. Blue: DAPI staining; Green: p65 (top panel) or NEMO (bottom panel). Images were taken at the magnification of 60x. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32201398), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] -
Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] - DDR & NF-kappa B are activated concomitantly in senescent MEFs & aged tissues. (A) Immunoblot detection of p-p65 & total p65 in liver tissue from 16-week-old WT (n=3) & Ercc1-/ delta (n=3) mice. (B) Immunoblot detection of phosphorylation of ATM & downstream targets gammaH2AX & p21 in liver from 16-week-old WT & Ercc1-/ delta mice. (C) Immunoblot detection of phosphorylation of NF-kappa B & I kappaB alpha in liver lysates from 3, 12 & 24 month-old WT mice. n=3 mice per group. (D) Immunoblot detection of p-ATM, ATM & p21 in the same liver lysates. (E) Immunoblot detection of DDR effectors in nuclear extracts from passage 5 WT & Ercc1-/- MEFs, grown at 20% oxygen. (F) Level of NF-kappa B activation is higher in Ercc1-/- MEFs compared to WT MEFs at passage 5, as measured by Immunoblot detection of p-p65 & total p65 in WT & Ercc1-/- MEFs at passage 5 after culturing in 20% oxygen. (G) Representative images of immunofluorescent detection of p65 & NEMO in passage 4 WT & Ercc1-/- MEFs grown at 20% oxygen. Blue: DAPI staining; Green: p65 (top panel) or NEMO (bottom panel). Images were taken at the magnification of 60x. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32201398), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] -
Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] - Induction of ubiquitin/proteasome-dependent DSBs in CPT-treated quiescent WI38 hTERT cells. (A–F) Serum-starved cells were treated with DMSO (1 h) or with MG132 (50 μM, 1 h), lactacystin (10 μM, 1 h), bortezomib (1 μM, 4 h), G5 (1.5 μM, 0.5 h) or Pyr-41 (9 μM, 0.5 h) before the addition of DMSO (untreated) or 25 μM CPT for 1 h & then co-stained for gammaH2AX (green) & 53BP1 (red) or analyzed by Western blot. ‘-’ in panels C & F means cells treated with DMSO. (A & D) Representative pictures. Nuclear contours, identified by DAPI staining (not shown), are indicated by dashed lines. Bars: 10 μm. (B & E) Number of gammaH2AX foci per nucleus from two independent experiments (147–153 nuclei were analyzed for each treatment). ****P < 0.0001. (C & F) Western blot of gammaH2AX. alphaTubulin: loading control. Dashed lines indicate that intervening wells have been spliced out. The top panels show quantification of gammaH2AX normalized to alphaTubulin (means ± SEM, n = 4 in panel C, n = 3 in panel F). ***P < 0.001; **P < 0.01. (G & H) Detection of DSBs by neutral Comet assays in serum-starved cells treated with DMSO or MG132 (25 μM) for 1 h before the addition of DMSO (untreated) or CPT for 1 h (7.5 μM for experiments (Exp) I & II; 5 & 7.5 μM for Exp III). (G) Representative pictures of nuclei from Exp I. (H) Quantification of neutral Comet tail moments for three independent experiments (95–133 nuclei were analyzed for each treatment in each experiment). ***P < 0.001; ****P < 0.0001. The untreated & CPT data from Exp I are from the same experiment as that of Supplementary Figure S3D. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26578593), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] -
Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] - Curcumin Analogs Induce Apoptosis in Cancerous Cells by Several Pathways. (a) E6-1 (Jurkat), dominant negative FADD (dnFADD) Jurkat, & overexpressing BCL-2 Jurkat were treated for 48 hours then stained for Annexin V & PI (b) E6-1 cells were plated & treated with or without the broad spectrum caspase inhibitor ZVAD(oMe)-FMK for 48 hours. Cells were stained for Annexin V & PI. Results were obtained using image-based cytometry with the Y-axis representative of percent of cells positive for Annexin V (green), PI (red), Annexin V & PI (yellow), or negative for both Annexin V & PI (blue). Values are expressed as a mean ± SD from three independent experiments. (c) E6-1 cells were treated for 24 hours with or without the broad spectrum caspase inhibitor ZVAD(oMe)-FMK & the studied compounds, lysed & subjected to Western blot analysis. (d) NHF & NCM460 cells were treated for 48 hours & 72 hours respectively, lysed & subjected to Western blot analysis. Bands were visualized with a chemiluminescence reagent. Images are representative of three independent experiments. Statistical calculations were performed using Two-Way ANOVA multiple comparison. *p < 0.05 vs % viable of Control (DMSO); #p < 0.05 vs % viable cells for groups without Z-VAD(oMe)FMK. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28439094), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] -
Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] - Curcumin Analogs Induce Apoptosis in Cancerous Cells by Several Pathways. (a) E6-1 (Jurkat), dominant negative FADD (dnFADD) Jurkat, & overexpressing BCL-2 Jurkat were treated for 48 hours then stained for Annexin V & PI (b) E6-1 cells were plated & treated with or without the broad spectrum caspase inhibitor ZVAD(oMe)-FMK for 48 hours. Cells were stained for Annexin V & PI. Results were obtained using image-based cytometry with the Y-axis representative of percent of cells positive for Annexin V (green), PI (red), Annexin V & PI (yellow), or negative for both Annexin V & PI (blue). Values are expressed as a mean ± SD from three independent experiments. (c) E6-1 cells were treated for 24 hours with or without the broad spectrum caspase inhibitor ZVAD(oMe)-FMK & the studied compounds, lysed & subjected to Western blot analysis. (d) NHF & NCM460 cells were treated for 48 hours & 72 hours respectively, lysed & subjected to Western blot analysis. Bands were visualized with a chemiluminescence reagent. Images are representative of three independent experiments. Statistical calculations were performed using Two-Way ANOVA multiple comparison. *p < 0.05 vs % viable of Control (DMSO); #p < 0.05 vs % viable cells for groups without Z-VAD(oMe)FMK. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28439094), licensed under a CC-BY license. Not internally tested by Novus Biologicals.











![Immunohistochemistry: Rabbit Polyclonal Histone H2AX [p Ser139] Antibody - Histone H2AX [p Ser139] Antibody](https://resources.bio-techne.com/images/products/antibody/nb100-384_rabbit-polyclonal-histone-h2ax-p-ser139-antibody-immunohistochemistry-16102023102936..png)
![Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] - Histone H2AX [p Ser139] Antibody](https://resources.bio-techne.com/images/products/nb100-384_rabbit-polyclonal-histone-h2ax-p-ser139-antibody-271220231285618.jpg)
![Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] - Histone H2AX [p Ser139] Antibody](https://resources.bio-techne.com/images/products/nb100-384_rabbit-polyclonal-histone-h2ax-p-ser139-antibody-310202415371991.jpg)
![Immunocytochemistry/ Immunofluorescence: Histone H2AX [p Ser139] Antibody [NB100-384] - Histone H2AX [p Ser139] Antibody](https://resources.bio-techne.com/images/products/nb100-384_rabbit-polyclonal-histone-h2ax-p-ser139-antibody-3102024153972.jpg)
![Immunocytochemistry/ Immunofluorescence: Histone H2AX [p Ser139] Antibody [NB100-384] - Histone H2AX [p Ser139] Antibody](https://resources.bio-techne.com/images/products/nb100-384_rabbit-polyclonal-histone-h2ax-p-ser139-antibody-310202415384166.jpg)
![Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] - Histone H2AX [p Ser139] Antibody](https://resources.bio-techne.com/images/products/nb100-384_rabbit-polyclonal-histone-h2ax-p-ser139-antibody-310202415304284.jpg)
![Immunocytochemistry/ Immunofluorescence: Histone H2AX [p Ser139] Antibody [NB100-384] - Histone H2AX [p Ser139] Antibody](https://resources.bio-techne.com/images/products/nb100-384_rabbit-polyclonal-histone-h2ax-p-ser139-antibody-310202415371942.jpg)
![Immunocytochemistry/ Immunofluorescence: Histone H2AX [p Ser139] Antibody [NB100-384] - Histone H2AX [p Ser139] Antibody](https://resources.bio-techne.com/images/products/nb100-384_rabbit-polyclonal-histone-h2ax-p-ser139-antibody-31020241534358.jpg)
![Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] - Histone H2AX [p Ser139] Antibody](https://resources.bio-techne.com/images/products/nb100-384_rabbit-polyclonal-histone-h2ax-p-ser139-antibody-3102024153565.jpg)
![Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] - Histone H2AX [p Ser139] Antibody](https://resources.bio-techne.com/images/products/nb100-384_rabbit-polyclonal-histone-h2ax-p-ser139-antibody-310202415525231.jpg)
![Immunohistochemistry: Histone H2AX [p Ser139] Antibody [NB100-384] - Histone H2AX [p Ser139] Antibody](https://resources.bio-techne.com/images/products/nb100-384_rabbit-polyclonal-histone-h2ax-p-ser139-antibody-310202416572.jpg)
![Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] - Histone H2AX [p Ser139] Antibody](https://resources.bio-techne.com/images/products/nb100-384_rabbit-polyclonal-histone-h2ax-p-ser139-antibody-310202415533820.jpg)
![Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] - Histone H2AX [p Ser139] Antibody](https://resources.bio-techne.com/images/products/nb100-384_rabbit-polyclonal-histone-h2ax-p-ser139-antibody-310202415541922.jpg)
![Immunocytochemistry/ Immunofluorescence: Histone H2AX [p Ser139] Antibody [NB100-384] - Histone H2AX [p Ser139] Antibody](https://resources.bio-techne.com/images/products/nb100-384_rabbit-polyclonal-histone-h2ax-p-ser139-antibody-310202415525243.jpg)
![Immunocytochemistry/ Immunofluorescence: Histone H2AX [p Ser139] Antibody [NB100-384] - Histone H2AX [p Ser139] Antibody](https://resources.bio-techne.com/images/products/nb100-384_rabbit-polyclonal-histone-h2ax-p-ser139-antibody-31020241682137.jpg)
![Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] - Histone H2AX [p Ser139] Antibody](https://resources.bio-techne.com/images/products/nb100-384_rabbit-polyclonal-histone-h2ax-p-ser139-antibody-3102024165718.jpg)
![Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] - Histone H2AX [p Ser139] Antibody](https://resources.bio-techne.com/images/products/nb100-384_rabbit-polyclonal-histone-h2ax-p-ser139-antibody-31020241682193.jpg)
![Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] - Histone H2AX [p Ser139] Antibody](https://resources.bio-techne.com/images/products/nb100-384_rabbit-polyclonal-histone-h2ax-p-ser139-antibody-310202415541946.jpg)
![Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] - Histone H2AX [p Ser139] Antibody](https://resources.bio-techne.com/images/products/nb100-384_rabbit-polyclonal-histone-h2ax-p-ser139-antibody-31020241612653.jpg)
![Western Blot: Histone H2AX [p Ser139] Antibody [NB100-384] - Histone H2AX [p Ser139] Antibody](https://resources.bio-techne.com/images/products/nb100-384_rabbit-polyclonal-histone-h2ax-p-ser139-antibody-31020241683932.jpg)