Detection of Human SOX2 by Western Blot
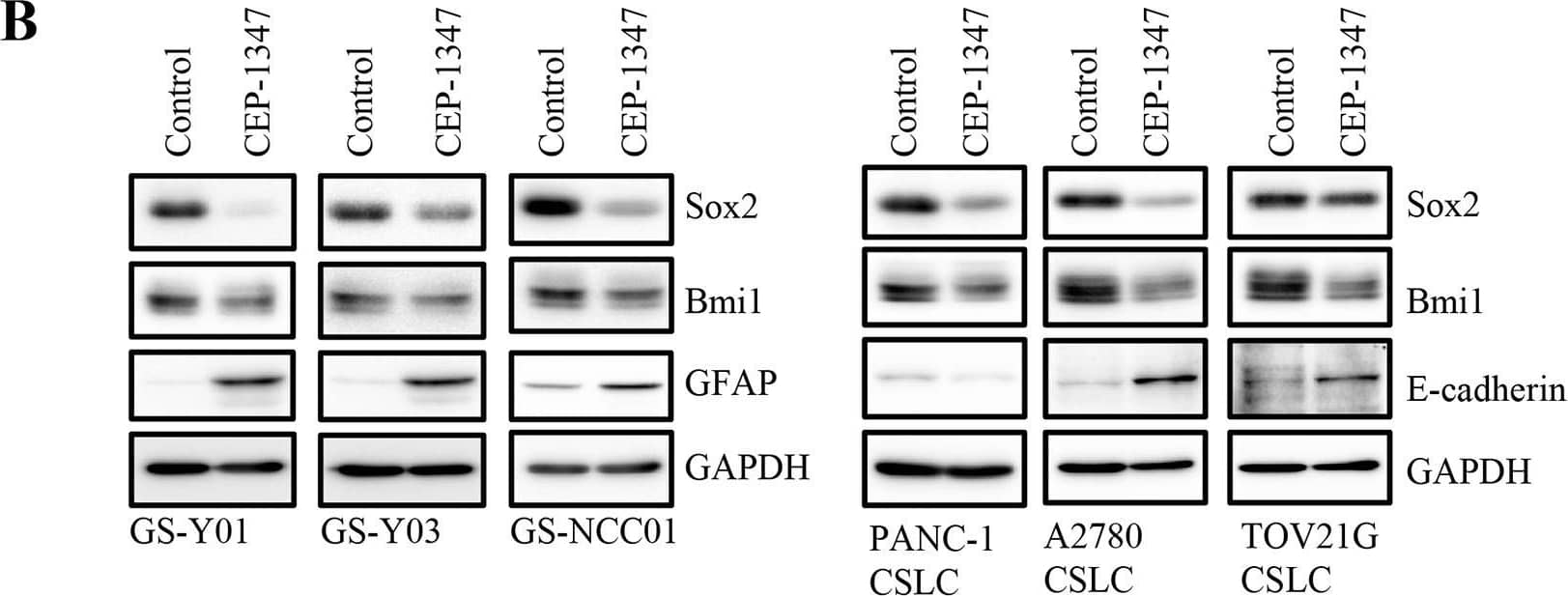
CEP-1347 induces the differentiation of cancer stem cells into non-cancer stem cells(A) Glioma stem cells (GS-Y01, GS-Y03, and GS-NCC01), pancreatic cancer stem cells (PANC-1 CSLC), and ovarian cancer stem cells (A2780 CSLC, and TOV21G CSLC) treated without (Control) or with CEP-1347 (300 nM for PANC-1 CSLC, 200 nM for the others) for 6 days were subjected to flow cytometric analysis of the cell-surface expression of CD133. Representative flow cytometric plots together with the percentages of CD133-positive cells are shown. (B) Cells treated as described in (A) were subjected to immunoblot analysis of the indicated proteins. (C) PANC-1 CSLC cells treated without (Control: lane 1) or with CEP-1347 (lane 2) for 6 days and those cultured in the presence of CEP-1347 for 6 days and then in its absence for the indicated days (lanes 3-5) were subjected to immunoblot analysis of the indicated proteins. Image collected and cropped by CiteAb from the following publication (https://www.oncotarget.com/lookup/doi/10.18632/oncotarget.22033), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Rat SOX2 by Immunocytochemistry/Immunofluorescence
Fluoxetine inhibits the differentiation of hypothalamic neuroprogenitor cells.Hypothalamic neuroprogenitor cells were differentiated for 18 days, in the presence or absence of fluoxetine (1 µM). Representative confocal photomicrographs for mature neurons marker Neu-N (green) (A). Fluoxetine decreases the percentage of Neu-N positive cells and the incubation with Trk receptors inhibitor K252a (24 h) partially reverses this effect (B). Representative confocal photomicrographs for the progenitor cells marker SOX-2 (green) (C). Fluoxetine increases the percentage of SOX-2 positive cells and the incubation with K252a (24 h) does not change this effect (D). Fluoxetine does not up regulate the levels of neurotrophic factor BDNF but incubation with K252a (24 h) results in a compensatory increase of BDNF mRNA levels (E). DAPI (blue), nuclear staining. Mean ± SEM; n = 4/5; One-Way ANOVA; ns, p>0.05; *, p<0.05; ***, p<0.001 compared to control; NS, p>0.05, #, p<0.05 compared to fluoxetine. Scale bar: 50 µm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24598761), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human SOX2 by Immunocytochemistry/Immunofluorescence
Hes3 is a marker of normal and cancer human stem cells.(A–C) Hes3+ cell in the striatum of non-cancerous adult human brain tissue (blood vessels identified by RECA-1 expression), human hemangioblastoma (HBM) biopsy (HBM Hes3+ megakaryocytes shown), human glioblastoma multiforme (GBM) biopsy (Hes3 co-expressed with prominin). (D) Fetal cortical cells sorted for prominin express Sox2 and Hes3. (E) Enrichment for Sox2+ and Hes3+ cells by magnetic sorting using an anti-prominin antibody. (Size bar, 50 µm). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/20195471), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human SOX2 by Immunocytochemistry/Immunofluorescence
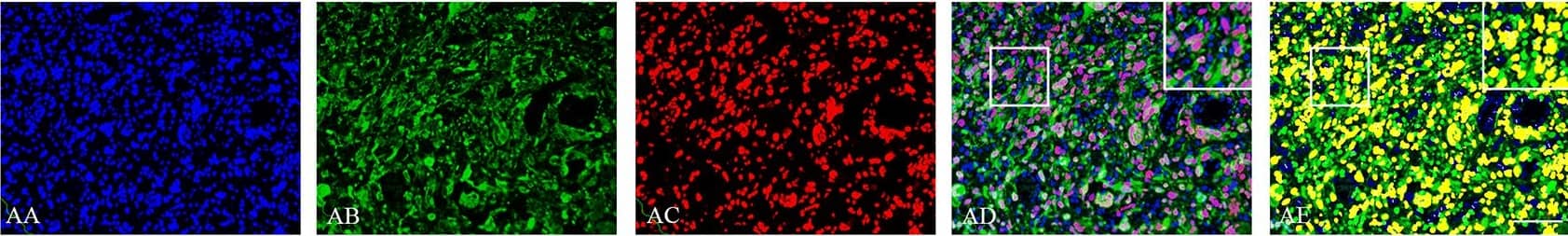
Double immunofluorescence staining of core and periphery of patient glioblastomas.Histological sections were stained with Dapi (blue), IDH1 (green) and Sox-2 (red) (AA-BE), Bmi-1 (red) (CA-DE) and Glut-3 (red) (EA to FE). The software-based classifier is shown in the right column. The classifier illustrates tumor cells co-expressing markers of interest in yellow and tumor cells not co-expressing markers of interest in green. The fluorescence stainings were quantified in both core and periphery for Sox-2 (G,J), Bmi-1 (H,K) and Glut-3 (I,L). Statistical comparison was performed using student’s t-test and ANOVA, ** p
Detection of Human SOX2 by Immunocytochemistry/Immunofluorescence
Immunohistochemistry of a parallel section of Figure 1. (a) Poor GFAP expression. (b) High Nestin expression. (c) SOX2 is highly expressed in nuclei of the external slope of circumscribed necrosis. (d) REST expression, all DAB. (e) CD133-positive area, frozen section. (f) Musashi-1-positive area. Scale bar 50 μm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/21869887), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human SOX2 by Immunocytochemistry/Immunofluorescence
Neural induction of hPSCs. (a) Both hPSC culture and neural differentiation were performed on LN521 substrate. Cortical neurons were induced with dual SMAD inhibition, expanded in the presence of FGF2, and matured with support by a selection of neurotrophic factors. Neural progenitor cells (NPCs) could be cryopreserved at day 21 and plated at day 32 for final experiments, including microelectrode array (MEA) measurements. (b) Three different hPSC lines (one hESC line, 08/023 and two hiPSC lines, 10212.EURCCs and IMR90-4) were characterized for their efficiency in producing neuroectodermal cells in response to 12-day neural induction by dual SMAD inhibition. Cells were stained for pluripotency marker Oct4 at the pluripotent stage and after 12 days of neural induction. The presence of early neuroectodermal markers was evaluated with Sox2, FoxG1 and Pax6 staining. (c) After 21 days of differentiation, the culture contained vimentin- and Pax6-positive NPCs that could be cryopreserved. Additionally, the first Tbr2- and MAP2-positive neurons were detected at this time point. (d) The percentages of Pax6-positive cells were quantified at four time points of differentiation (mean ± s.e.m., n = 5–14, data derived from 1–3 independent differentiations). Statistical analysis was performed with the Mann-Whitney U test to compare differences between day 12 and day 46 within each hPSC line, and significant p-values are presented in the image. The scale bar is 50 µm in all images. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31748598), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human SOX2 by Immunocytochemistry/Immunofluorescence
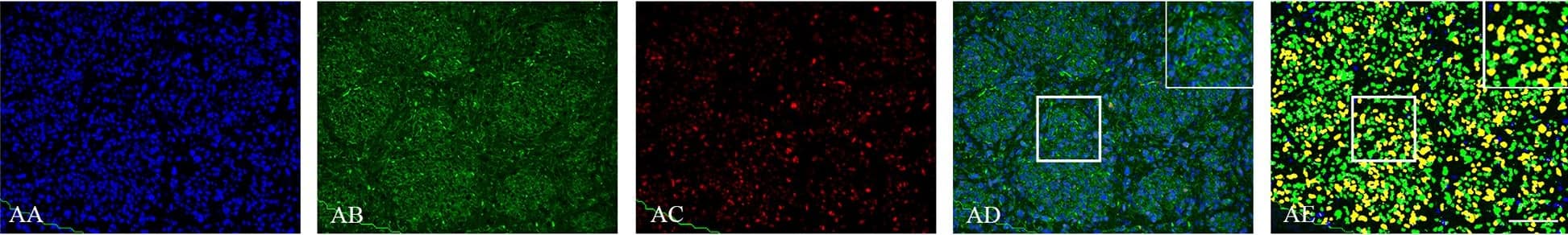
Double immunofluorescence staining of core and periphery in orthotopic model with five different patient derived GBM spheroid cultures.Tissues were stained with Dapi (blue), Vimentin (green) and Sox-2 (red), Bmi-1 (red) and Glut-3 (red). The software-based classifier is shown in the right column. The classifier illustrates tumor cells co-expressing markers of interest in yellow and tumor cells not co-expressing markers of interest in green. The fluorescence stainings were quantified in both central part and periphery for Sox-2 (G), Bmi-1 (H) and Glut-3 (I). Statistical comparison was performed using student’s t-test. Scalebar: 200μm. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0155106), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human Human/Mouse/Rat SOX2 Antibody by Immunocytochemistry/ Immunofluorescence
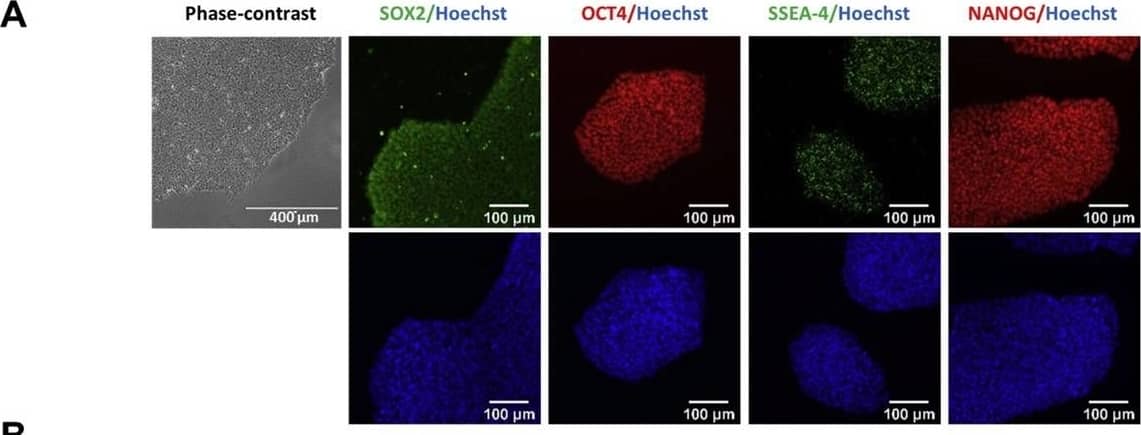
Characterization of TRNDi009-C iPSC line. A) Left: phase contrast imaging of TRNDi009-C colonies grown on Geltrex at passage 6. Right: Representative immunofluorescent images of iPSCs positive for stem cell markers: SOX2, OCT4, NANOG, and SSEA4. Nucleus is labelled with Hoechst 33342 (blue). B) Cytogenetic analysis showing a normal karyotype (46, XX). C) Detection of heterozygous mutation of a p.L302P (c.905 T > C) in exon 2 of the SMPD1 gene. D) Flow cytometry analysis of pluripotency protein markers: NANOG and TRA-1–60. E) RT-PCR verification for the clearance of the Sendai virus from reprogrammed cells. Sendai virus vector transduced fibroblasts were used as a positive control. F) Pathological analysis of teratoma from TRNDi009-C iPSC, showing a normal ectodermal, mesodermal, and endodermal differentiation. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31132580), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human Human/Mouse/Rat SOX2 Antibody by Immunocytochemistry/ Immunofluorescence
Characterization of TRNDi007-B iPSC line.A) Left: Phase contrast imaging of TRNDi007-B colonies. Right: Representative immunofluorescent images of iPSCs showing expression of stem cell markers: SOX2, OCT4, SSEA-4, and NANOG. Nucleus is stained with Hoechst dye (in blue). B) Flow cytometry analysis of pluripotency protein markers: TRA-1-60, NANOG and SSEA-4. C) Cytogenetic analysis showing a normal karyotype (46, XY). D) Detection of homozygous mutation of c.2560 C > T in exon 18 of the GAA gene. E) RT-PCR verification of the clearance of Sendai virus from the reprogrammed cells. Sendai virus vector transduced fibroblasts was used as positive control. F) Histological analysis of teratomas produced by TRNDi007-B iPSCs. Representative images showing the presence of ectodermal, endodermal and mesodermal derivatives. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31026687), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human Human/Mouse/Rat SOX2 Antibody by Western Blot
CEP-1347 induces the differentiation of cancer stem cells into non-cancer stem cells(A) Glioma stem cells (GS-Y01, GS-Y03, and GS-NCC01), pancreatic cancer stem cells (PANC-1 CSLC), and ovarian cancer stem cells (A2780 CSLC, and TOV21G CSLC) treated without (Control) or with CEP-1347 (300 nM for PANC-1 CSLC, 200 nM for the others) for 6 days were subjected to flow cytometric analysis of the cell-surface expression of CD133. Representative flow cytometric plots together with the percentages of CD133-positive cells are shown. (B) Cells treated as described in (A) were subjected to immunoblot analysis of the indicated proteins. (C) PANC-1 CSLC cells treated without (Control: lane 1) or with CEP-1347 (lane 2) for 6 days and those cultured in the presence of CEP-1347 for 6 days and then in its absence for the indicated days (lanes 3-5) were subjected to immunoblot analysis of the indicated proteins. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/29212273), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human SOX2 by Immunocytochemistry/ Immunofluorescence
(A) Differentiation markers for the endoderm (FOXA2, PDX1), mesoderm ( alpha-actin, brachyury), and ectoderm ( beta3 Tubulin, Otx2) were detected for the MUCG01, MUCG02, and MUCG03 clinical-grade hESC lines by immunocytochemistry. (B) Standard karyotypes without chromosomal aberration were detected for all hESC lines MUCG01: 46,XX; MUCG02: 46,XX; and MUCG03: 46,XY. (C) Pluripotency markers Oct3/4, Sox2, and Nanog were detected for the MUCG01, MUCG02, and MUCG03 clinical-grade hESC lines by immunocytochemistry. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36293356), licensed under a CC-BY license. Not internally tested by R&D Systems.