Human PP1 alpha Catalytic Subunit Antibody
R&D Systems, part of Bio-Techne | Catalog # MAB6105

Key Product Details
Species Reactivity
Validated:
Cited:
Applications
Validated:
Cited:
Label
Antibody Source
Product Specifications
Immunogen
Gly304-Arg317
Accession # P62136
Specificity
Clonality
Host
Isotype
Scientific Data Images for Human PP1 alpha Catalytic Subunit Antibody
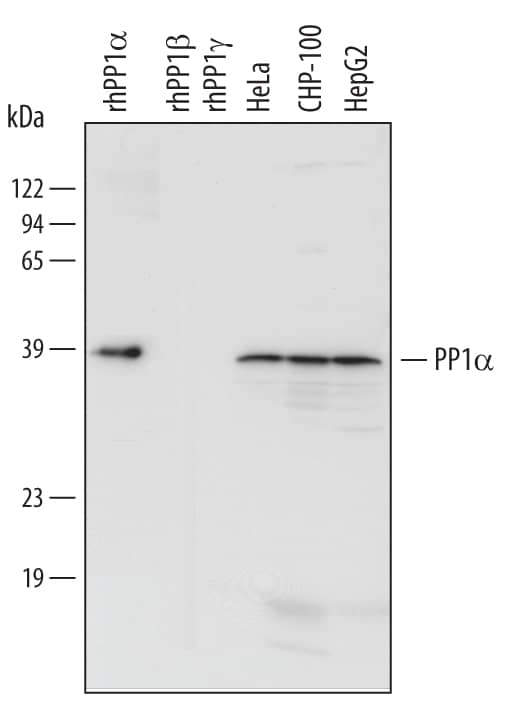
Detection of Human PP1 alpha by Western Blot.
Western blot shows lysates of HeLa human cervical epithelial carcinoma cell line, CHP-100 human neuroblastoma cell line, and HepG2 human hepatocellular carcinoma cell line. PVDF Membrane was probed with 1 µg/mL of Human PP1 alpha Catalytic Subunit Monoclonal Antibody (Catalog # MAB6105) followed by HRP-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # HAF007). For additional reference, recombinant human PP1 alpha, recombinant human PP1 beta, and recombinant human PP1 gamma (5 ng/lane) were included. A specific band was detected for PP1 apha at approximately 39 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 3.Detection of Human PP1 alpha/PPP1A by Western Blot
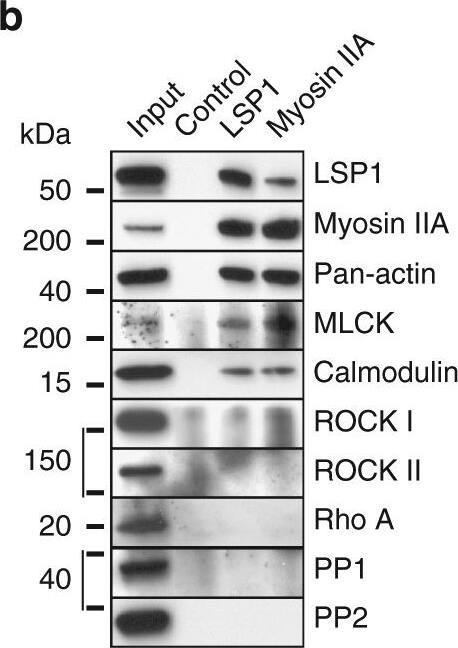
LSP1 and supervillin interact with a similar subset of myosin IIA regulators. a Western blots of macrophage lysates progressively extracted by increased concentration of TritonX-100 (1–5%), or in RIPA buffer. Note that LSP1, and also myosin IIA, are mostly extracted in the 2% and RIPA fractions. b Western blot of immunoprecipitation of endogenous LSP1 or myosin IIA from macrophage lysates, with control IgG and input. Blots were probed with indicated antibodies. Note cross-coprecipitation of LSP1 and myosin IIA, accompanied by coprecipitation of myosin regulators MLCK and calmodulin. Molecular weight in kDa is indicated. c Western blots of anti-GFP immunoprecipitation of lysates from macrophages expressing LSP1 full length or LSP1 domains fused to GFP (For inputs, see Supplementary Fig. 7A). Blots were probed with indicated antibodies. Note coprecipitation of myosin IIA with full length LSP1, and also with the C-terminal and the villin headpiece-like domains (V1V2) of LSP1. Interestingly only the full length and the C-terminal constructs, but not V1V2, are also able to bind MLCK, pMLC and calmodulin. An LSP1-positive band in the lane of the C-terminal construct probably reflects the fact that the anti-LSP1 antibody recognizes an epitope in the C-terminal half of LSP1. d Immunoprecipitation of myosin IIA from macrophage lysates, with addition of Mg2+/ATP and/or latrunculin A (10 µM), as indicated. Upper panel: colloidal Coomassie stained SDS PAGE gel, lower panels: corresponding western blots developed with indicated antibodies. Molecular weight is indicated in kDa. e Quantification of coprecipitated amounts of actin and LSP1, normalized to precipitations performed without addition of Mg2+/ATP and/or latrunculin A. f Western blots of anti-GFP immunoprecipitations from lysates of macrophages expressing LSP1-GFP or supervillin construct SV1-830-GFP developed with indicated primary antibodies. Molecular weight in kDa is indicated. Note that LSP1 and SV1-830 coprecipitate comparable amounts of myosin IIA, MLCK and calmodulin, but that supervillin-coprecipitated myosin is more activated, as indicated by pMLC signal Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/s41467-018-02904-x), licensed under a CC-BY license. Not internally tested by R&D Systems.Applications for Human PP1 alpha Catalytic Subunit Antibody
Western Blot
Sample: HeLa human cervical epithelial carcinoma cell line, CHP‑100 human neuroblastoma cell line, and HepG2 human hepatocellular carcinoma cell line
Formulation, Preparation, and Storage
Purification
Reconstitution
Formulation
Shipping
Stability & Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: PP1 alpha
Protein Phosphatase 1, also known as PP1 and PPP1, is an enzyme that removes phosphate groups attached to serine or threonine residues in proteins. The holoenzyme is composed of two subunits, a catalytic subunit that is highly conserved throughout evolution, and a wide variety of regulatory subunits that target the enzyme to specific subcellular compartments and proteins. Three isoforms of the catalytic subunit are ubiquitously expressed in tissues and cell lines: PP1 alpha, PP1 beta and PP1 gamma.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional PP1 alpha Products
Product Documents for Human PP1 alpha Catalytic Subunit Antibody
Product Specific Notices for Human PP1 alpha Catalytic Subunit Antibody
For research use only